The Adaptive Immune Response in Graves’ Disease: Does Vitamin D have a role?
Dyah Purnamasari,1 Pradana Soewondo,1 Samsuridjal Djauzi2
Dyah Purnamasari, MD
Division of Endocrinology, Department of Medicine
Faculty of Medicine, University of Indonesia
Cipto Mangunkusumo General Hospital
Salemba 6, Jakarta 10430, Indonesia
Tel. No.: +62213100073
Fax No.: +62213928658/59
E-mail: dyah_p_irawan@yahoo.com
e-ISSN 2308-118x
Printed in the Philippines
Copyright © 2014 by the JAFES
Received October 17, 2013. Accepted May 7, 2014.
Graves' disease (GD) is an autoimmune disease characterized by excessive autoantibody formation by the lymphocyte B cells (B cells). The auto antibodies will bind to Thyroid Stimulating Hormone receptors (TSHR) and enhance the production of thyroid hormone. Previous studies indicate that the impairment of immune response in GD happens in several points in the adaptive immune response, particularly the profile of the intrathyroidal dendritic cells (tDC), the imbalance of T helper-1 (Th1) and T helper-2 (Th2), the Th17 cells that act as pro-inflammatory cells and the dysfunction of immune modulating T regulator (Treg) cells.6-11
Vitamin D is a steroid hormone which has pleiotropic effects. The role of vitamin D in bone and calcium metabolism is already established. The discovery of vitamin D receptor (VDR) in immune cells such as monocytes/macrophages, T cells and B cells,demonstrates that vitamin D may influence innate and adaptive immune process. Recent studies try to explore the relationship between vitamin D and autoimmune disease, furthermore they consider vitamin D as a modifiable environmental factor in autoimmune diseases.13,40 Most people with autoimmune diseases have lower vitamin D level than that of healthy subjects. Vitamin D level also has been associated with disease activity of Systemic Lupus Erythematosus (SLE) and Rheumatoid Arthritis (RA).
Vitamin D influences adaptive immune response through its ability to modulate dendritic Cells (DC), T cells, B cells and Treg cells. Although previous studies reported the immune response disturbance in GD include the tDC, Thelper and Treg cells, 6-11 little is known whether the immunoregulatory effect of vitamin D can interfere with the natural history of GD. The effect of vitamin D in GD remains to be explored.
Key words: Graves’ Disease, adaptive immune response, Vitamin D
The Adaptive Immune Response in Graves’ Disease: Does Vitamin D have a role?
Dyah Purnamasari,1 Pradana Soewondo,1 Samsuridjal Djauzi2
Dyah Purnamasari, MD
Division of Endocrinology, Department of Medicine
Faculty of Medicine, University of Indonesia
Cipto Mangunkusumo General Hospital
Salemba 6, Jakarta 10430, Indonesia
Tel. No.: +62213100073
Fax No.: +62213928658/59
E-mail: dyah_p_irawan@yahoo.com
e-ISSN 2308-118x
Printed in the Philippines
Copyright © 2014 by the JAFES
Received October 17, 2013. Accepted May 7, 2014.
Graves' disease (GD) is an autoimmune disease characterized by excessive autoantibody formation by the lymphocyte B cells (B cells). The auto antibodies will bind to Thyroid Stimulating Hormone receptors (TSHR) and enhance the production of thyroid hormone. Previous studies indicate that the impairment of immune response in GD happens in several points in the adaptive immune response, particularly the profile of the intrathyroidal dendritic cells (tDC), the imbalance of T helper-1 (Th1) and T helper-2 (Th2), the Th17 cells that act as pro-inflammatory cells and the dysfunction of immune modulating T regulator (Treg) cells.6-11
Vitamin D is a steroid hormone which has pleiotropic effects. The role of vitamin D in bone and calcium metabolism is already established. The discovery of vitamin D receptor (VDR) in immune cells such as monocytes/macrophages, T cells and B cells,demonstrates that vitamin D may influence innate and adaptive immune process. Recent studies try to explore the relationship between vitamin D and autoimmune disease, furthermore they consider vitamin D as a modifiable environmental factor in autoimmune diseases.13,40 Most people with autoimmune diseases have lower vitamin D level than that of healthy subjects. Vitamin D level also has been associated with disease activity of Systemic Lupus Erythematosus (SLE) and Rheumatoid Arthritis (RA).
Vitamin D influences adaptive immune response through its ability to modulate dendritic Cells (DC), T cells, B cells and Treg cells. Although previous studies reported the immune response disturbance in GD include the tDC, Thelper and Treg cells, 6-11 little is known whether the immunoregulatory effect of vitamin D can interfere with the natural history of GD. The effect of vitamin D in GD remains to be explored.
Key words: Graves’ Disease, adaptive immune response, Vitamin D
Graves' disease (GD) is an autoimmune disease that mostly affects individuals within their reproductive age. The pathophysiology of GD has not yet been fully explained. Similar to other autoimmune diseases, the autoimmune process in Graves' disease is initiated by the failure of tolerance for autoantigens which triggers a series of adaptive immune responses. The result is an excessive formation of autoantibodies.3-5 Several animal and human studies show there are some changes in adaptive immune responses in GD including the dominance of the TH2 pathway, the increase of pro-inflammatory TH 17 cells, the decrease of T regulator cells and the increased activity of intrathyroidal dendritic cells (tDC).6-11
Associated with this immune response, vitamin D is a hormone that has a non-classical effect as an immunomodulator.12-14 Biological effects of vitamin D are mediated by the vitamin D receptor (VDR). VDR is found on immune cells including DC, lymphocytes, macrophages and NK cells.15-16 The enzyme vitamin D 1α-OHase, that converts vitamin D 25 (OH) into its active form vitamin D 1,25 (OH), is also borne by cells/organs other than the kidney. Since immune cells also use this enzyme, vitamin D could potentially influence the immune response in autoimmune diseases 14,16-17
The role of vitamin D in autoimmune diseases is still controversial.18 A possible relationship starts from epidemiological findings that autoimmune diseases are more often found in low latitude areas with low sunlight exposure. Populations with autoimmune diseases tend to have lower level of vitamin D than that of normal population. Experimental animal and human studies regarding vitamin D therapy on autoimmune disease also provide controversial results. In rheumatoid arthritis and SLE, vitamin D therapy reduces the incidence and disease activity. Vitamin D therapy in Crohn's disease lowers the activity of DC.13, 18-19
Although the profile of the adaptive immune response in Graves' disease has been reported several times,6-11 the role of vitamin D in adaptive immune response in GD is unknown. Studies of vitamin D on GD reported by Yasuda and Yamashita reports that the concentration of vitamin D in GD is lower than normal subjects. Furthermore, the concentration of vitamin D in intractable GD were lower than that of remission GD. 20-22 It is not known whether vitamin D levels influence the occurrence or remission state of GD. This paper will talk about the immunological profile of GD and the possible role of vitamin D as an immunomodulator .
Click here to download Figure 1
Figure 1. Stages of vitamin D status29

The figure explains the relationship between vitamin D concentrations with its functional ability in the absorption of calcium. The concentration of vitamin D 25 (OH) <12.5 nmol/l (<5 ng/dl) define vitamin D deficiency. The optimal functional capability of vitamin D 25 (OH) will be achieved at concentrations close to 75 nmol/l (30 ng/dl).
A meta-regression analysis showed vitamin D 25(OH) level approaching insufficiency criteria i.e., 54 ± 1.3 nmol/l globally. Based on the incidence of secondary hyperparathyroidism, vitamin D 25(OH) level <50 nmol/l denotes insufficiency and vitamin D 25(OH) level <25 nmol/l denotes deficiency.
The level of vitamin D 25(OH) in Northern Europe and Southeast Asia are the highest, and the lowest levels are in Latin America and Southern Europe.30 In South Asia, the prevalences of vitamin D insufficiency range from 78 to 96%, and in Southeast Asia, the prevalences of vitamin D deficiency range from 47 to 92%. The mean vitamin D level among premenopausal women in Indonesia and Malaysia is 48 nmol/l (equivalent to 19.2 ng/dl).31
Vitamin D is a hormone that has pleiotropic effects. Aside from its role in calcium and bone metabolism, vitamin D also possesses non-classic al effects in the cardiovascular, reproductive and immune system. Most of the biological activities of 1,25(OH)2D3 are mediated by the Vitamin D Receptor (VDR), a high-affinity receptor that acts as a ligand-activated transcription factor. The immuno-modulatory effects of vitamin D begins with the discovery of VDR on immune cells such as dendritic cells (DCs), macrophages and lymphocytes. In addition, immune cells also have 1α-hydroxylase vitamin D which can convert vitamin D 25(OH) into the active form, vitamin D 1,25(OH)2.13-14,32 The ability of the immune cell to convert vitamin D 25(OH) into the active form is an autocrine mechanism that maintains their regulatory functions and demonstrates the importance of vitamin D adequacy in the immune system.14
Vitamin D 1,25(OH)2 may increase the antimicrobial property of monocytes and macrophages by increasing the ability of their chemotactic and phagocytic process. Monocytes and macrophages are able to respond to pathogen-associated molecular patterns (PAMPs) of several types of infections as well as pattern-recognition receptors such as Toll-like receptors (TLRs) and they are the first defense mechanism against a microbial attack. In addition, through VDR, vitamin D 1,25 (OH) 2 also increases the regulation of cathelicidin HCAP-18 gene. 32
The role of vitamin D in enhancing the innate immune response is demonstrated in the elimination process of Mycobacterium tuberculosis (M.TBC). This process is initiated by TLR activation of monocytes and macrophages which then increases the expression of VDR gene and other genes that lead to induction of cathelicidin antimicrobial peptide (CAMP).32 Corollary to this, during the wound healing process, the level of CYP27B1, which converts vitamin D 25(OH) into vitamin D 1,25(OH)2, increases in the wound. It stimulates the expression of cathelicidin, TLR2 and CD14 and activates the innate immune response.13
Aside from stimulating the antimicrobial activities of immune cells, vitamin D 1,25(OH)2 also inhibits the expression of TLR2 and TLR4 in monocytes, thus reducing their response to PAMP. The mechanism will disappear after 72 hours of microbial exposure, and serve as a negative feedback mechanism to prevent excessive TLR stimulation in the later stages of infection.32
In the adaptive immune response, vitamin D influences DC, T cells and B cells. The dendritic cell, the main target of 1,25(OH)2D, is a potent antigen-presenting cell (APC) which is capable of capturing, processing, and presenting antigen to T cells. Vitamin D Receptor signaling pathway activation inhibits DC maturation as evidenced by decreased levels of DC marker, MHC-class II, costimulatory molecules (CD40, CD80, CD86) as well as other maturation induced surface markers (CD83). Research on human blood donors showed that 1,25(OH)2D prevents differentiation of immature DC (iDC) and mature DC (mDC) from monocytes during monocyte derived dendritic cell (MDDC) culture under stimulation of GM-CSF and IL-4 (growth factors for differentiation of MDDC). Alteration during MDDC process is characterized by down-regulation of costimulatory molecules, (CD40, CD80 and CD86), and MHC-class II molecules that reduces the ability of DC as APC to activate T cells. Furthermore, 1,25(OH)2D also supports the spontaneous apoptosis of mature DC. Briefly, the immunosuppressive effects of 1.25(OH)2D are through inhibition of differentiation, maturation, activation and disturbance in DC that causes a decrease in T cell responses.13,33-34 The differentiation of monocytes (DC precursors) into macrophages or DC is followed by decreasing VDR expression so that the mature DC become less sensitive to the effect of vitamin D 1,25 (OH). The mechanism aims to balance the activity of immune response.32,35
Vitamin D 1,25(OH)2 also regulates the expression of DC-derived chemokines and cytokines, which inhibits the production of IL-12 and IL-23 (primary cytokines in the differentiation of Th1 and Th17 pathway), increases the release of IL-10 (cytokines with broad anti-inflammatory activity) and increases release of chemokines MIP-3a/CCLL22 (chemokines which play a role in the recruitment of CCR4 expressing Treg). In addition, by modulating DC-derived cytokines, 1,25(OH)2D influences the balance of Th-derived cytokines by inhibiting the production of inflammatory Th1 (IFN-γ, IL-2) and Th17 (IL-17 and IL21) pathways and promotes the Th2 phenotype.32
With respect to tolerance and the autoimmune process, 1,25(OH)2D can enhance Treg cells and promotes tolerogenic DC and then influences the tolerance process in transplantation and prevents the development of autoimmune diseases.13 Jeffery et al., investigated the role of 1,25(OH)2D on Treg cells and proinflammatory cytokines using healthy donor blood cultures. They showed that stimulation of 1,25(OH)2D on Treg inhibits the production of several cytokines such as IFN-γ, IL-21 and IL-17. Also, 1,25(OH)2D stimulates the expression of FoxP3 and CTLA-4 on Treg cells with the help of IL-2. The addition of IL-2 alone in the culture medium did not significantly increase the expression of Foxp3. But, if the addition of IL-2 is accompanied by 1,25(OH)2D, there was a sharp increase (two fold) in the expression of Foxp3 and CTLA-4 in Treg cells. It showed that 1,25(OH)2D and IL-2 have a synergistic effect on activated T cells, serves as a powerful anti-inflammatory, and can stimulate physiological adaptive Treg cells.36
Boonstra did an experimental animal study using naive CD4 Mel14 T cells with plate bound antiCD3 and soluble anti-CD28 mAb in the presence or absence of vitamin D3. The cells were repeatedly stimulated, and their cytokines developed into a highly polarized Th2 population when cultured with vitamin D3, similarly to cultures with APC. Although APC is the immunomodulatory target of vitamin D3, vitamin D3 can also directly stimulate the TCR, influence the polarization of naive T cells and then inhibit Th1 pathway (IFN-γ production) and increased Th2 pathway (IL-4, IL-5 and IL-10). This is the first study that shows the direct effect of vitamin D3 on Th cells in the absence of APC.37
Mahon showed that the targets of vitamin D depends on the differentiation and activation status of T-cells. Since naive T cells have little VDR, they are relatively unresponsive to vitamin D 1,25(OH)2 in the first 24 hours of activation.38
Although T cell activation will stimulate B cells, 1,25(OH)2D can directly affect B cells through the inhibition of the proliferation and differentiation of plasma cells, the inhibition of Ig secretion, the improvement of B cell memory and the stimulation of B cell apoptosis. A study by Heine using healthy donor blood cultures showed that B cells can regulate immune response by producing 1,25 (OH)2D through an autocrine mechanism as well as increase the expression of IL-10 by B cells alone, T cells and DC. IL-10 is a pleiotropic cytokine that inhibits antigen presentation by DCs and macrophages and also inhibits T cell activation. In addition, IL-10 inhibits the migration of B cells and stimulates them to change into plasmablasts.32,39
Click here to download Figure 2
Figure 2. The role of vitamin D 1,25(OH)2 in innate and adaptive immune response32
Adapted from Baeke F. Current Opinion in Pharmacology 2010
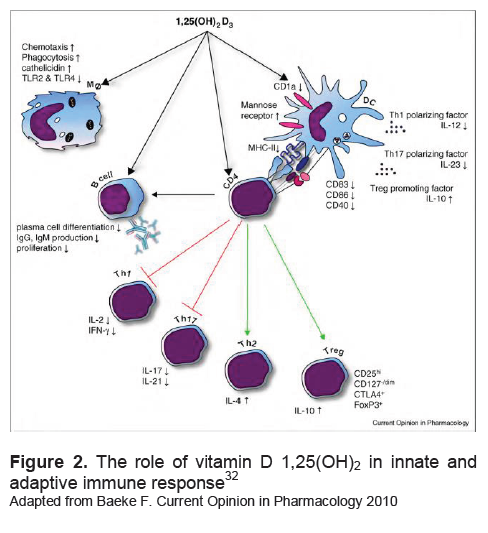
Vitamin D 1,25(OH)2 influence innate immune cells and adaptive immune cells. In innate immune cells, vitamin D would improve the ability of phagocytosis and chemotaxis and decrease the expression of TLR-2 and TLR-4. Vitamin D also prevents the excessive production of proinflammatory cytokines. To the adaptive immune cells, vitamin D is able to decrease the expression of costimulatory molecules, MHC-II and the cytokine IL-12, as well as improve the function of T reg cells by increasing cytokine IL-10. Vitamin D also has a direct effect on CD4 T cells to shift the immune response to the Th2 pathway and Treg.
Although immune cells have VDR and enzyme 1α-OH-ase, the role of vitamin D in autoimmune disease is still controversial.13,40 Epidemiologic study demonstrated the effect of vitamin D deficiency on the incidence of multiple sclerosis (MS), diabetes mellitus type 1 (DM1), inflammatory bowel disease (IBD), rheumatoid arthritis (RA) and systemic lupus erythematosus (SLE).27,31
In observational ecologic studies, some autoimmune diseases such as IBD, MS, RA are more common in the northern latitudes which have low sunlight exposure. Besides that, MS has a correlation with winter births (maternal vitamin D status during pregnancy is low) and disease recurrence is more frequent in winter.23,40-41
Prospective studies showed that 25(OH)D levels are associated with RA incidence, DM1 and MS. It is not clear whether a low concentration of vitamin D is the result of autoimmune disease or is a cause of autoimmune disease.41 A systematic review reported the association between vitamin D and the risk of autoimmune disease and effects of vitamin D supplementation on their clinical presentation. The study showed vitamin D could prevent the incidence of DM1. Physiopathologic studies show that among individuals who have a genetic risk factor, hypovitaminosis D can impair tolerance.41-43
The role of oral supplementation of vitamin D in autoimmune disease was also reported in SLE, RA and IBD.44 Studies in Malang, Indonesia, showed the serum vitamin D 25(OH) level of SLE patients were significantly lower than that of healthy subjects (22.80 ± 16:23 ng/dl vs. 35.15 ± 7.61 ng/dl, p = 0:00). As many as 55.5% of SLE patients have vitamin D deficiency and the levels are inversely correlated with antibody level (anti-ds-DNA and anti-vitamin D).45 A systematic review showed that vitamin D can improve several sign and symptoms of the disease, but is not necessarily related to the severity of the disease.42
The role of vitamin D on DC profile in autoimmune diseases has not been widely reported. Handono reported a negative correlation between vitamin D 25(OH) level with the expression of DC maturation markers (CD11c +, CD40, CD83 and CD86) in SLE.35
In Crohn's disease, the intervention of vitamin D 25(OH) or vitamin D 1,25(OH)2 in monocyte-derived dendritic cells culture (MDDC) impairs the maturation of DC, which is characterized by decreased expression of HLA-DR molecules, CD80, CD83, CD86 and increased expression of CD14. In addition to this, intervention of vitamin D in vitro also decreased the production of proinflammatory cytokines such as tumor necrosis factor-α (TNF-α), IL-12 and IL-10 but elevates IL-6.46 Aside from the vitro study, Bartels also gave vitamin D,1200 IU/day of vitamin D3 for six months to Crohn's disease patients. Before and after administration of oral vitamin D3, he analyzed DC maturation and cytokine production. Oral administration of vitamin D impairs the maturation of DC shown by decreasing expression of CD80 molecules.19 Besides impairing the expression of CD80, the concentration of inflammatory cytokines, TNF-α and IFN-γ, also decline.19,46 Vitamin D intervention on DC maturation has also been reported in SLE patients, it decreased the expression of HLA-DR, CD40 and CD86.47
Graves' disease is characterized by excessive autoantibodies that bind to the TSHR in thyroid cells and stimulates thyroid hormone production resulting in hyperthyroidism.5,48 It is the most common cause of hyperthyroidism (60-80%), affecting mainly women aged 40 to 60 years. The pathogenesis is still not fully understood but genetic predisposition and environmental factors leads to disruption tolerance to self-antigens and triggers an autoimmune thyroid disease AITD.49
Under normal conditions, the immune response will protect the body from foreign proteins and is able to tolerate self-antigens through central and peripheral tolerance. The first defense mechanism against autoimmunity is that not every Ag is able to stimulate T cells, but only Ag presented by specific MHC molecules.
Central tolerance occurs in the thymus gland, if the body eliminates autoreactive T cells and T cells that interact with immature DC. However, not all T cells will be destroyed in the thymus, some will escape into the peripheral circulation.
In peripheral tolerance, at steady state (prior to infection and inflammation), DCs are in the immature state and will be able to recognize self-antigens or foreign weak Ag (protein) and create tolerance to the Ag. This stage is important because the DC should be able to distinguish between self-antigens and foreign potent Ag. When there is an infection or inflammation, the environment around the DC changes, with an increase of proinflammatory cytokines that stimulates the maturation of DC. Once mature DC develop, the potential self antigens can potentially trigger adaptive immune responses against self antigens (autoimmunity). However, if the tolerance to self-antigens have occurred prior to the infection, the immune response to self-antigens can be prevented. The failure of tolerance to self-antigens will induce autoimmune diseases.50-51
DC maturation plays an important role in the process of tolerance and autoimmunity by changing the nature of the iDC, which has ability to capture antigens but are not immunogenic, into DC which have weak endocytic ability but are immunogenic [DCs are able to stimulate effector T cells (CD4, CD8) and raise the production of cytokines that supports a subsequent immune response (IFN-γ)].51 Beside inducing tolerance, in vitro studies showed that repeated stimulation of T cells with iDC will develop a population of T cell that produce IL-10 (Treg cells). Based on the description above, the iDC plays an important role in central and peripheral tolerance.52-53
The initial immune response in GD requires both a specific and non-specific signal. Ag-specific signals are exogenous or endogenous, whereas the non-specific signals are costimulatory signals and inflammatory cytokines. Endogenous sources can be the dead-thyrocyte, whereas exogenous signals can be either viral or bacterial. The TSH receptor undergoes a posttranslational split, forming 2 subunit structures with subunit A serving as a self antigen. The adaptive immune response in GD starts with the presentation of this specific Ag signal (exogenous or endogenous) by DCs, to T cells.48,54 In the early stages, T cell receptors (TCR) on the surface of T cells interacts with MHC-peptide complexes on the APC. After this stage, naive T cells require subsequent signals to activate processes.
The next stage is the interaction between CD28 on the T cell surface and costimulatory molecules CD80/CD86 on the APC. The existence of two signals (TCR with MHC-peptide and CD28 with CD 80/CD86) will activate naive T cells and subsequent immune response that lead to the formation of antibodies by B cells. 55
In addition to potent APC, the autoimmune process is also triggered by environmental factors, including immune cell-produced by cytokines due to Ag stimulation. The cytokines (IL-1β, TNF-α, IL-12, IFN-γ, IFN-α, IL-13, etc.) will increase the efficiency of the antigen presentation by increasing the expression of MHC and costimulatory molecules on APC. If there are no inflammatory cytokines, the costimulatory signal appears weak or absent, then the antigen presentation is suboptimal and induces the tolerance process.48
Kabel et al., compared the profile of tDC in GD and in normal thyroid glands. The study involved thyroid tissues of 13 GD patients who underwent surgery and 9 normal thyroid tissue (6 specimens were obtained at laryngectomy (laryngeal carcinoma and 3 specimens were obtained from autopsy). They were able to show normal human thyroid glands contain fewer population of mononuclear cell (MNC) which reacted strongly positive for class II MHC markers, similar with the morphologic characteristic of DC. This MNC did not react to RFD1 and L25 markers (markers characteristic of DC on secondary lymph organs which are actively involved in immune response). These findings suggest that there is a small number of DC in normal thyroid gland but they are not actively involved in the immune response and in antigen handling. The tDC serve to remove foreign objects and materials that have been damaged by the thyroid to the thyroid-draining lymph nodes. In thyroid glands of GD, there are more tDC and they react more positively to markers RFD1 and L25, which were shown to be actively involved in the immune response including autoantigen handling. Some tDC are even seen to interact with lymphocytes in the thyroid gland. The results of this study correlated with a previous animal study on laboratory rats, were the number of tDC was increased and it preceded the appearance of anticolloid antibodies in the circulation.11 The DC are found outside the thyroid gland, in draining lymph nodes around the thyroid and lymph vessels. A series of general immunological processes follows: tDC captures antigen (as immature DC) and brings the antigen to the lymph nodes for presentation to T cells (transform to mature DC). The migration process of mature DC is followed by the expansion and maturation of T cells and B cells in the lymph channels, even infiltrating the thyroid gland.48
Fourteen years later, Quadbeck examined several types of DC in Graves’ thyroid glands. Quadbeck’s study involved thyroid tissues of 15 GD patients who underwent thyroid surgery. The results showed that there were three populations of t-DC: 1) Immature t-DC (MHC II+/CD40-/CD80-) found in perifollicular thyroid tissue, 2) Partially mature CD80+ t-DC located in connective tissue and interstitial clusters, and 3) Mature t-DC (MHC II+/ CD40+/CD80+/RFD1+) were in clusters and adjacent/interacting with activated Th cells (CD4+/MHC-II+). Immature t-DC were also found in venous capillaries. The number of immature t-DC in GD patients were higher than that of healthy subjects and toxic adenoma (toxic goiter, TG), respectively: 95%; 55%; and 51%. It means that the t-DC in some GD patients undergo maturation in clusters (groups) which resemble lymphoid tissue. The maturation of t-DC in these clusters is also supported by the intimate contact between t-DC and CD4+/MHC class II+ Th cells. The expression of HLA-DR by thyrocytes were elevated, which might indicate the cooperation between DC, macrophages and thyrocytes in triggering autoimmunity in GD.10
The role of DC and the association between DC and Treg in GD was investigated by Mao et al., involving 77 untreated Graves' disease (uGD) 13 euthyroid patients with ATD (euthyroid graves' disease, eGD), 15 patients with euthyroid Hashimoto's thyroiditis (euthyroid Hashimoto, eHT) and 74 healthy controls. The study showed that the proportion of Treg cells in the uGD is lower (1.57±0.67% vs 3.36±0.96%, p<0.001) than that of the healthy controls, but not in the eGD group (2.98±0,88%) and eHT (3.09±1.02%). Although the number was lower, the function of T-reg cells in uGD was maintained. Moreover, the proportion of T-reg cells was also negatively correlated with TSHR antibody level in the uGD (r=-0.735, p<0.001). The proportion of costimulatory molecules CD86, CD80 and CD40 increased in the uGD when compared with the control group. The proportion of pDC in the uGD was significantly higher than that of control group (41.95±8.39% vs 32.85±8.02%, p<0.001), eGD group (31.63±7.07%, p<0.001) and HT group (28.50±3.96%, p<0.0001). Furthermore, the pDC/DC ratio in the uGD was the highest and the ratio of pDC/DC is negatively related to the proportion of T-reg cells (r=-0.689, p<0.001). This phenomenon showed that there was polarization of pDC among uGD that might play a role in reducing the proportion of T-reg cells. Since plasmacytoid DC (pDC) is a major producer of IFN-α, the increased proportion of pDC will raise the cytokines IFN-α in the uGD. The T-reg cells undergoing apoptosis in the uGD with IFN-α dependent mechanism.
GD patients have predominant Th2 cytokines and increased Th17 cells. The Th2 pathway will secrete IL-4 that will activate B cell-producing antibodies. 54 The imbalance of Th1 and Th2 pathways in GD patients is demonstrated by several studies that investigated the Th1 and Th2 cells derived cytokines, either in the thyroid gland or peripheral blood vessels. To see the differences of intrathyroidal cytokine mRNA production between autoimmune and non-autoimmune thyroid disorders, Heuer et al., analyzed cytokine mRNA expression levels in thyroid tissue samples from 13 GD subjects, 2 HT subjects, 5 multinodular nontoxic goiter (MNNT) subjects and 4 TA subjects. The GD patients are divided into two groups based on the titer of antibody as follows: anti-thyroperoxidase (anti-TPO) > 4000 U/mL called GD high and anti-TPO < 200 U/mL called GD low. The results showed that the GD high group had higher IL-4 mRNA concentration as much 2-4 times more than that of among GD low group. Furthermore, the concentration of IL-10 mRNA among GD high group were significantly higher than that of other groups. The concentrations of IFN-γ, IL-1/3, IL-8 and CD25 mRNA among GD high group were also significantly higher than those in the GD low group. The highest level of IFN-γ, IL-2 and CD25 mRNA were found among the HT group, while the lowest level of mRNA were found among the TA group. The study showed that among GD high group, there was a shift to Th2 pathway, whereas among HT group the Th1 pathway was pronounced which indicate the dominant T cell mediated cytotoxic process.6
Phenekos et al., documented the pattern of immune response in GD by measuring both Th1 and Th2 serum cytokine profiles among patients suffering from autoimmune disease and toxic nodular goiter. The study involved 25 normal subjects (control) and 43 patients. Twenty-five patients suffered from GD, 21 from HT and 7 from toxic nodular goiter. Measured serum cytokines by enzyme-linked immunosorbent assay (ELISA) included: IL-2, IL-1β, IFN-α, TNF-β, IL-12, IL-5, IL-10, IL-8, IL-4 and IL-5. The study showed that GD subjects had a higher level of IL-4 and IL-5 than those of the other groups, while the HT subjects had a higher level of IL-2, IFN-γ, IL-12 and IL-18 than those of the other groups. It means that GD subjects had dominant Th2 cytokine pathway, whereas HT subjects had dominant Th1 cytokine pathway.9
The role of Th1/Th2 pathway mechanism in the pathogenensis of GD was also examined in experimental animals by Nagayama et al. Hyperthyroidism is induced by using an adenovirus that expresses human TSHR A subunit. The study showed that the disruption of IFN-γ and IL-4 in BALB/c mice will prevent hyperthyroidism.7
In addition to the proportion of Th1 and Th2 cytokine profile, Nanba et al., reported that there was an increase of Th17 cells expression among GD patients which has proinflammatory activity. The study involved 17 patients with Hashimoto's thyroiditis (Hashimoto thyroiditis, HT) who received thyroxine (severe HT), 17 patients with Hashimoto 's thyroiditis without thyroxine (mild HT), 18 euthyroid GD with anti-thyrotropin receptor antibody (TRAb) positive after 5 years of anti-thyroid drug therapy (intractable GD), 17 euthyroid GD patients with negative TRAb for more than 2 years after stopping anti-thyroid drug treatment (GD in remission) and 10 control subjects. The study reported that the proportion of Th2 cells among the intractable GD group and mild HT were significantly higher than that of the control group. The ratio of Th1/Th2 cells in severe HT was higher than that of mild HT. The proportion of Th17 cells in patients with intractable GD and GD remission were higher than that of control group. Furthermore, the proportion of Th17 cells in intractable GD group was higher than that of GD with remission.8
The relationship between vitamin D status with GD is not widely reported. From the point of view of genetic studies, a meta-analysis reported an association between VDR polymorphisms with GD in Asian populations, but not in the Caucasian population.57
The Yamashita study in Japan involving 208 GD patients showed that the prevalence of vitamin D deficiency among female GD reached 39.7%, it was higher than that of males, by as much as 17.8%. This prevalence is influenced by seasonal change, the highest prevalence is 60%, during April to June; the lowest prevalence is less than 20%, during July-September. The mean level of vitamin D in men and women GD subjects men and women respectively were 41.3±15.0 and 31.8±13.3 nmol/l. This value is lower than that of a previous study by Kobayashi, who examined 758 healthy subjects in Japan whose mean concentration of 25(OH)D was 59.4 nmol/l (~23.76 ng/ml). Females aged 20-29 years had the lowest vitamin D level and the highest prevalence of vitamin D deficiency. Low levels of vitamin D among young women can be triggered by avoiding sun exposure and the use of sunscreen; men tend to spend more time engaged in outdoor activities.20,43
Eleven years later, after Yamashita's study, Yasuda conducted a study in the same place among 26 female naive GD and 46 healthy female subjects, to investigate the effect of vitamin D level on the volume of thyroid gland. They found the mean level of vitamin D among GD was significantly lower than that of the control group (14.4±4.9 vs 17.1±4.1 ng/ml, p<0,05). Vitamin D level is associated with thyroid volume (r=0.45, p<0.05) but not with thyroid function and the TRAb level. Yasuda also investigated the difference in vitamin D level between remission GD and non-remission GD. The study involved 18 remission GD, 36 non-remission GD and 49 control subjects. Vitamin D level among non-remission GD is significantly lower than that of remission GD and the control group (14.5±2.9 vs 18.2±5.1 vs 18.6±5.3 ng/ml). Among GD, vitamin D level does not change due to treatment with ATD. 41-42
Cross sectional studies showed there is an association between vitamin D level and GD, including the remission status, however, there is no large prospective study that investigates the effects of vitamin D status on the onset and clinical remission of GD.21,58 In animal studies, Misharin investigated the relationship between vitamin D deficiency and hyperthyroidism induced in BALB/c mice. The study demonstrated that an environmental factor, vitamin D, has only minor effects on induced immunity to the TSHR but that it directly affects thyroid function in mice.59
Click here to download Figure 3
Figure 3. The estimation diagram about the immuno-modulatory effects of vitamin D in GD. Legend: TSHR (Thyroid Stimulating Hormone Receptor); T4 (Thyroxin); T3 (Triiodothyronin); DC (Dendritic Cell); MHC (Major Histocompatibility Complex); LPS (Lipopolysaccharide); APC (Antigen Presenting Cells); TCR (T Cell Receptor)

The role of vitamin D supplementation on clinical improvement of untreated hyperthyroid patients was reported by Tani in Japan. The 24-week clinical trial involves 30 patients, and aims to study the effects of vitamin D 1,25(OH)2 on the decline of thyroid hormone levels in 30 hyperthyroid patients. The subjects were randomly divided into two groups, the first group received 30 mg of MMI, while the second group receive the same dose of MMI with added vitamin D 1,25(OH)2. During the monitoring period, the total doses of MMI did not differ between the two groups. The levels of FT3, fT4, T3 and T4 decreased significantly and more rapidly in the second group, as well as did the increase in TSH level. The level of TRAb did not differ between the two groups. The study showed the benefits of vitamin D 1,25 (OH)2 supplementation in GD patients but the mechanism was still unknown at that time. However, in experimental animal studies in the same year on the effect of vitamin D on thyrocytes, it may be that he beneficial effects of vitamin D in hyperthyroid patients may be due to a direct effect on the thyrocytes. It is not known whether the clinical improvement is associated with the role of vitamin D in adaptive immune response in GD. It should be explored whether vitamin D influences the maturation of DCs and the activation of T cell or B cell.60
Theoretically, the effects of vitamin D as an immunomodulator influences the tolerance process which consists of the maturation of DC and induction of Treg cell. Impaired immune response in GD may include the activity of APC (DC), the polarization of Th1/Th2 and downregulation of Treg cells. However, whether vitamin D system affects the natural history of GD, still needs to be proven.
Adaptive immune response in GD starts with the stimulation of mature DC with self antigen (TSHR peptide) and activation T helper cell. T helper cell will stimulate B cell to produce autoantibody. Vitamin D may alter the series of immune process in GD through several ways. First, vitamin D will reduce the maturation of DC; second, vitamin D will directly suppressed the Th cell activation; third, vitamin D will hamper the production of autoantibody by the B cell and the fourth, vitamin D will stimulate Treg cell to suppress the activation of Th cells and B cells.
1. Weetman AP. Graves' disease. N Engl J Med. 2000;343(17):1236-48.
2. Weetman AP, McGregor AM, Hall R. Evidence for an effect of antithyroid drugs on the natural history of Graves' disease. Clin Endocrinol (Oxf). 1984;21:163-72.
3. Cooper DS. Hyperthyroidism. The Lancet. 2003;362:459-68.
4. Orgiazzi J. Thyroid autoimmunity. Presse Med. 2012;41:e611-25.
5. Swain M, Swain T, Mohanty BK. Autoimmune thyroid disorders-An update. Indian Journal of Clinical Biochemistry. 2005;21(1):9-17.
6. Heuer M, Aust G, Ode-Hakim S, Scherbaum WA. Different cytokine mRNA profiles in Graves' disease, Hashimoto's thyroiditis, and nonautoimmune thyroid disorders determined by quantitative reverse transcriptase polymerase chain reaction (RT-PCR). Thyroid. 1996;6(2):97-106.
7. Nagayama Y, Saitoh O, Mclachlan SM, Rapoport B, Kano H, Kumazawa Y. TSH receptor-adenovirus-induced Graves' hyperthyroidism is attenuated in both interferon-γ and interleukin-4 knockout mice; implication for the Th1/Th2 paradigm. Clin Exp Immunol. 2004;138:417-22.
8. Nanba T, Watanabe M, Inoue N, Iwatani Y. Increases of the Th1/Th2 cell ratio in severe Hashimoto's disease and in the proportion of Th17 cells in intractable Graves' disease. Thyroid. 2009;19(6):495-.
9. Phenekos C, Vryonidou A, Gritzapis AD, Baxevanis CN, Goula M, Papamichail M. Th1 and Th2 serum cytokine profiles characterize patients with Hashimoto's thyroiditis (Th1) and Graves' disease (Th2). Neuroimmunomodulation. 2004;11:209-13.
10. Quadbeck B, Eckstein AK, Tews S, Walz M, Hoermann R, Mann K, et al. Maturation of thyroidal dendritic cells in Graves' Disease. Scand J Immunol. 2002;55:612-20.
11. Kabel PJ, Voorbij HAM, Haan MD, Gaag RDVD, Drexhage HA. Intrathyroidal dendritic cells. J Clin Endocrinol Metab. 1988;65:199-207.
12. Adams JS, Liu PT, Chun R, Modlin RL, Hewison M. Vitamin D in the defense of the human immune response. Ann NYAcadSci. 2007;1117:94-105.
13. Adorini L, Penna G. Control of autoimmune diseases by the vitamin D endocrine system. Nature Clinical Practice Rheumatology. 2008;4(8):404-12.
14. Aranow C. Vitamin D and the immune system. J Investig Med. 2011;59(6):881-6.
15. Brown AJ, Dusso A, Slatopolsky E. Vitamin D. Am J Physiol Renal Physiol. 1999;277:157-75.
16. DeLuca H. Overview of general physiologic features and functions of vitamin D. Am J Clin Nutr. 2004;80:1689s-96s.
17. Etten Ev, Mathieu C. Immunoregulation by 1,25-dihydroxyvitamin D3: Basic concepts. Journal of Steroid Biochemistry & Molecular Biology. 2005;97:93-101.
18. Cantorna MT, Mahon BD. Mounting evidence for vitamin D as an environmental factor affecting autoimmune disease prevalence. Experimental Biology and Medicine. 2004;229:1136-42.
19. Bartels LE, Bendix M, Hvas CL, Jorgensen SP, Agnholt J, Agger R, et al. Oral vitamin D3 supplementation reduces monocyte-derived dendritic cell maturation and cytokine production in Crohn's disease patients. Inflammopharmacol. 2013.
20. Yamashita H, Noguchi S, KeisukeTakatsu, Koike E, Murakami T, Watanabe S, et al. High prevalence of vitamin D deficiency in Japanese female patients with Graves' disease. Endocrine Journal. 2001;48(1):63-9.
21. Yasuda T, Okamoto Y, Hamada N, Miyashita K, Takahara M, Sakamoto F, et al. Serum vitamin D levels are decreased in patients without remission of Graves' disease. Endocrine. 2013;43:230-2.
22. Yasuda T, Okamoto Y, Hamada N, Miyashita K, Takahara M, Sakamoto F, et al. Serum vitamin D levels are decreased and associated with thyroid volume in female patients with newly onset Graves' disease. Endocrine. 2012;42:739-41.
23. Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357:266-81.
24. Dusso AS, Brown AJ, 2005;289:8-28. SEAJPRP. Vitamin D. Am J Physiol Renal Physiol. 2005;289:8-28.
25. Kulie T, Groff A, Redmer J, Hounshell J, S S. Vitamin D: An evidence-based review. J Am Board Fam Med. 2009:698-706.
26. Zittermann A. Vitamin D in preventive medicine: Are we ignoring the evidence? British Journal of Nutrition. 2003;89:552-72.
27. Li J, Byrne ME, Chang E, Jiang Y, Donkin SS, Buhman KK ea-. 1, 25-dihidroxyvitamin D hidroxylase in adipocytes. J Steroid BiochemMol Biol. 2008;112:122-6.
28. Ogunkolade BW, Boucher BJ, Prahl JM, Bustin SA, Burrin JM, Noonan K ea-. Vitamin D receptor (VDR) mRNA and VDR protein levels in relation to vitamin D status, insulin secretory capacity and VDR genotype in Bangladeshi Asians. . Diabetes. 2002;51:2294-312.
29. Yetley E. Assessing the vitamin D status of the US population. Am J ClinNutr 2008;88:558S-64S.
30. Hagenau T, Vest R, Gissel TN, Poulsen CS, Erlandsen M, Mosekilde L, et al. Global vitamin D levels in relation to age, gender, skin pigmentation and latitude: An ecologic meta-regression analysis. Osteoporos Int. 2009;20:133-40.
31. Mithal A, Wahl DA, Bonjour JP, Burckhardt P, Dawson-Hughes B, Eisman JA, et al. Global vitamin D status and determinants of hypovitaminosis D. Osteoporosis Int. 2009;20:1807-20.
32. Baeke F, Takiishi T, Korf H, Gysemans C, Mathieu C. Vitamin D: Modulator of the immune system. Current Opinion in Pharmacology. 2010;10:482-96.
33. Berer A, Stockl J, Majdic O, Wagner T, Kollars M, Lechner K, et al. 1,25-dihydroxyvitamin D3 inhibits dendritic cell differentiation and maturation in vitro. Experimental Hematology. 2000;28:575-83.
34. Piemonti L, Monti P, Marina S, Fraticelli P, Leone BE, Cin ED, et al. Vitamin D3 affects differentiation, maturation and function of human monocyte-derived dendritic cells. Journal of Immunology. 2000;164:4443-51.
35. Handono K, Daramatasia W, Pratiwi, Sunarti S, Wahono S, Kalim H. Low level of vitamin D increased dendritic cell maturation and expression of interferon-γ and interleukin-4 in systemic lupus erythematosus. Journal of pharmacy and biological sciences. 2012;2(4):37-43.
36. Jeffery LE, Burke F, Mura M, Zheng Y, Qureshi OS, al. HMe. 1,25-dihydroxyvitamin D3 and interleukin-2 combine to inhibit T cell production of inflammatory cytokines and promote development of regulatory T cells expressing CTLA-4 and FozP3. J Immunol 2009;183:5458-67.
37. Boonstra A, Barrat FJ, Crain C, Heath VL, Savelkoul HFJ, O'Garra A. 1α,25-dihydroxyvitamin D3 has a direct effect on naive CD4+ to enhance the development of Th2 cells. The Journal of Immunology. 2001;167:4974-80.
38. Mahon BD, Wittke A, Weaver V, Cantorna MT. The targets of vitamin D depend on the differentiation and activation status of CD4+ T cells. J Cell Biochem. 2003;89:922-32.
39. Heine G, Niesner U, Chang H-D, Steinmeyer A, Zugel U, Zuberbier T, et al. 1,25-dihydroxyvitamin D3 promotes IL-10 production in human B cells. Eur J Immunol. 2008;38:2210-8.
40. Schwalfenberg GK. Solar radiation and vitamin D: Mitigating environmental factors in autoimmune disease. Journal of Environmental and Public Health. 2012:1-9.
41. Kriegel MA, Manson JE, Costenbader KH. Does vitamin D affect risk of developing autoimmune disease?: A sytematic review. Semin Arthritis Rheum. 2011;40:512-31.
42. Antico A, Tampoia M, Tozzoli R, Bizzaro N. Can supplementation with vitamin D reduce the risk or modify the course of autoimmune disease? A systematic review of the literature. Autoimmune Rev. 2012;12(2):127-36.
43. Holick MF. Sunlight and vitamin D for bone health and prevention ofautoimmune disease, cancers and cardiovascular disease. Am J Clin Nutr 2004;80:1678s-88s.
44. Leventis P, Patel S. Clinical aspects of vitamin D in the management of rheumatoid arthritis. Rheumatology. 2008;47:1617-21.
45. Handono K, Gani AA, Ekawati M, Wahono S. Serum level of vitamin D and autoantibodies level in systemic lupus erythematosus (SLE) patients. Journal of Pharmacy and Biological Sciences. 2012;3(4):16-20.
46. Bartels LE, Jogersen SP, Bendix M, Hvas CL, Agnholt J, Agger R, et al. 25-Hydroxy vitamin D3 modulates dendritic cell phenotype and function in Crohn’s disease. Inflammopharmacol. 2013;21:177-86.
47. Ben-Zvi I, Aranow C, Mackay M, Stanevsky A, Kamen DL, Marinescu LM, et al. The impact of vitamin D on dendritic cell function in patients with systemic lupus erythematosus. Plos ONE. 2010;5(2):1-8.
48. Vasu C, Holterman MJ, Prabhakar B. Modulation of dendritic cell function and cytokine production to prevent thyroid autoimmunity. Autoimmunity. 2003;36(6-7):389-96.
49. Kawashima A, Tanigawa K, Akama T, Yoshihara A, Ishii N, Suzuki K. Innate immune activation and thyroid autoimmunity. J Clin Endocrinol Metab. 2011;96:3661-71.
50. Tsokos GC, Goust J-M, Virella G. Tolerance and autoimmunity. In: Virella G, editor. Medical Immunology. 5th ed. New York: Marcel Dekker; 2001. p. 313-40.
51. Steinman RM, Hawiger D, Liu K, Bonifaz L, Bonnyay D, Mahnke K, et al. Dendritic cell function in vivo during the steady state: A role in peripheral tolerance. Ann N Y Acad Sci. 2003;987:15-25.
52. Moser M. Antigen presentation, dendritic cells and autoimmunity In: Rose NR, Mackay IR, editors. The Autoimmune Disease. 4th ed. UK: Elsevier Academic Press; 2006. p. 37-46.
53. Shortman K, Liu Y-J. Mouse and human dendritic cell subtypes. Nature Reviews. 2002;2:151-61.
54. Prabhakar BS, Bahn RS, Smith TJ. Current perspective on the pathogenesis of Graves’ disease and ophthalmopathy. Endocrine Reviews 2003;24(6):802-35.
55. Weetman AP, DeGroot LJ. Autoimmunity to the thyroid gland. Thyroid Manager. 2013:1-127.
56. Mao C, Wang S, Xiao Y, Xu J, Jiang Q, Jin M, et al. Impairment of regulatory capacity of CD4+CD25+ regulatory T cells mediated by dendritic cell polarization and hyperthyroidism in Graves' disease. The Journal of Immunology. 2011;186:4734-43.
57. Zhou H, Xu C, Gu M. Vitamin D receptor (VDR) gene polymorphisms and Graves' disease: A meta-analysis. Clin Endocrinol (Oxf). 2009;70(6):938-45.
58. Pantazi H, PD P. Changes in parameters of bone and mineral metabolism during therapy for hyperthyroidism. J Clin Endocrinol Metab. 2000;85:1099-106.
59. Misharin A, Hewison M, Chen C-R, Lagishetty V, Aliesky HA, Mizuturi Y, et al. Vitamin D deficiency modulates Graves' hyperthyroidism induced in BALB/c mice by thyrotropin receptor immunization. Endocrinology. 2009;150(2):1051-60.
60. Kawakami-Tani T, EtsushiFukawa, Tanaka H, Abe Y, Makino I. Effect of lα-Hydroxyvitamin D3 on serum levels of thyroid bormones in hyperthyroid patients with untreated Graves' Disease. Metabolism. 1997:1184-118.
Authors are required to accomplish, sign and submit scanned copies of the JAFES Declaration that the article represents original material that is not being considered for publication or has not been published or accepted for publication elsewhere.
Consent forms, as appropriate, have been secured for the publication of information about patients; otherwise, authors declared that all means have been exhausted for securing such consent.
The authors have signed disclosures that there are no financial or other relationships that might lead to a conflict of interest. All authors are required to submit Authorship Certifications that the manuscript has been read and approved by all authors, and that the requirements for authorship have been met by each author.
Graves' disease (GD) is an autoimmune disease that mostly affects individuals within their reproductive age. The pathophysiology of GD has not yet been fully explained. Similar to other autoimmune diseases, the autoimmune process in Graves' disease is initiated by the failure of tolerance for autoantigens which triggers a series of adaptive immune responses. The result is an excessive formation of autoantibodies.3-5 Several animal and human studies show there are some changes in adaptive immune responses in GD including the dominance of the TH2 pathway, the increase of pro-inflammatory TH 17 cells, the decrease of T regulator cells and the increased activity of intrathyroidal dendritic cells (tDC).6-11
Associated with this immune response, vitamin D is a hormone that has a non-classical effect as an immunomodulator.12-14 Biological effects of vitamin D are mediated by the vitamin D receptor (VDR). VDR is found on immune cells including DC, lymphocytes, macrophages and NK cells.15-16 The enzyme vitamin D 1α-OHase, that converts vitamin D 25 (OH) into its active form vitamin D 1,25 (OH), is also borne by cells/organs other than the kidney. Since immune cells also use this enzyme, vitamin D could potentially influence the immune response in autoimmune diseases 14,16-17
The role of vitamin D in autoimmune diseases is still controversial.18 A possible relationship starts from epidemiological findings that autoimmune diseases are more often found in low latitude areas with low sunlight exposure. Populations with autoimmune diseases tend to have lower level of vitamin D than that of normal population. Experimental animal and human studies regarding vitamin D therapy on autoimmune disease also provide controversial results. In rheumatoid arthritis and SLE, vitamin D therapy reduces the incidence and disease activity. Vitamin D therapy in Crohn's disease lowers the activity of DC.13, 18-19
Although the profile of the adaptive immune response in Graves' disease has been reported several times,6-11 the role of vitamin D in adaptive immune response in GD is unknown. Studies of vitamin D on GD reported by Yasuda and Yamashita reports that the concentration of vitamin D in GD is lower than normal subjects. Furthermore, the concentration of vitamin D in intractable GD were lower than that of remission GD. 20-22 It is not known whether vitamin D levels influence the occurrence or remission state of GD. This paper will talk about the immunological profile of GD and the possible role of vitamin D as an immunomodulator .
Click here to download Figure 1
Figure 1. Stages of vitamin D status29

The figure explains the relationship between vitamin D concentrations with its functional ability in the absorption of calcium. The concentration of vitamin D 25 (OH) <12.5 nmol/l (<5 ng/dl) define vitamin D deficiency. The optimal functional capability of vitamin D 25 (OH) will be achieved at concentrations close to 75 nmol/l (30 ng/dl).
A meta-regression analysis showed vitamin D 25(OH) level approaching insufficiency criteria i.e., 54 ± 1.3 nmol/l globally. Based on the incidence of secondary hyperparathyroidism, vitamin D 25(OH) level <50 nmol/l denotes insufficiency and vitamin D 25(OH) level <25 nmol/l denotes deficiency.
The level of vitamin D 25(OH) in Northern Europe and Southeast Asia are the highest, and the lowest levels are in Latin America and Southern Europe.30 In South Asia, the prevalences of vitamin D insufficiency range from 78 to 96%, and in Southeast Asia, the prevalences of vitamin D deficiency range from 47 to 92%. The mean vitamin D level among premenopausal women in Indonesia and Malaysia is 48 nmol/l (equivalent to 19.2 ng/dl).31
Vitamin D is a hormone that has pleiotropic effects. Aside from its role in calcium and bone metabolism, vitamin D also possesses non-classic al effects in the cardiovascular, reproductive and immune system. Most of the biological activities of 1,25(OH)2D3 are mediated by the Vitamin D Receptor (VDR), a high-affinity receptor that acts as a ligand-activated transcription factor. The immuno-modulatory effects of vitamin D begins with the discovery of VDR on immune cells such as dendritic cells (DCs), macrophages and lymphocytes. In addition, immune cells also have 1α-hydroxylase vitamin D which can convert vitamin D 25(OH) into the active form, vitamin D 1,25(OH)2.13-14,32 The ability of the immune cell to convert vitamin D 25(OH) into the active form is an autocrine mechanism that maintains their regulatory functions and demonstrates the importance of vitamin D adequacy in the immune system.14
Vitamin D 1,25(OH)2 may increase the antimicrobial property of monocytes and macrophages by increasing the ability of their chemotactic and phagocytic process. Monocytes and macrophages are able to respond to pathogen-associated molecular patterns (PAMPs) of several types of infections as well as pattern-recognition receptors such as Toll-like receptors (TLRs) and they are the first defense mechanism against a microbial attack. In addition, through VDR, vitamin D 1,25 (OH) 2 also increases the regulation of cathelicidin HCAP-18 gene. 32
The role of vitamin D in enhancing the innate immune response is demonstrated in the elimination process of Mycobacterium tuberculosis (M.TBC). This process is initiated by TLR activation of monocytes and macrophages which then increases the expression of VDR gene and other genes that lead to induction of cathelicidin antimicrobial peptide (CAMP).32 Corollary to this, during the wound healing process, the level of CYP27B1, which converts vitamin D 25(OH) into vitamin D 1,25(OH)2, increases in the wound. It stimulates the expression of cathelicidin, TLR2 and CD14 and activates the innate immune response.13
Aside from stimulating the antimicrobial activities of immune cells, vitamin D 1,25(OH)2 also inhibits the expression of TLR2 and TLR4 in monocytes, thus reducing their response to PAMP. The mechanism will disappear after 72 hours of microbial exposure, and serve as a negative feedback mechanism to prevent excessive TLR stimulation in the later stages of infection.32
In the adaptive immune response, vitamin D influences DC, T cells and B cells. The dendritic cell, the main target of 1,25(OH)2D, is a potent antigen-presenting cell (APC) which is capable of capturing, processing, and presenting antigen to T cells. Vitamin D Receptor signaling pathway activation inhibits DC maturation as evidenced by decreased levels of DC marker, MHC-class II, costimulatory molecules (CD40, CD80, CD86) as well as other maturation induced surface markers (CD83). Research on human blood donors showed that 1,25(OH)2D prevents differentiation of immature DC (iDC) and mature DC (mDC) from monocytes during monocyte derived dendritic cell (MDDC) culture under stimulation of GM-CSF and IL-4 (growth factors for differentiation of MDDC). Alteration during MDDC process is characterized by down-regulation of costimulatory molecules, (CD40, CD80 and CD86), and MHC-class II molecules that reduces the ability of DC as APC to activate T cells. Furthermore, 1,25(OH)2D also supports the spontaneous apoptosis of mature DC. Briefly, the immunosuppressive effects of 1.25(OH)2D are through inhibition of differentiation, maturation, activation and disturbance in DC that causes a decrease in T cell responses.13,33-34 The differentiation of monocytes (DC precursors) into macrophages or DC is followed by decreasing VDR expression so that the mature DC become less sensitive to the effect of vitamin D 1,25 (OH). The mechanism aims to balance the activity of immune response.32,35
Vitamin D 1,25(OH)2 also regulates the expression of DC-derived chemokines and cytokines, which inhibits the production of IL-12 and IL-23 (primary cytokines in the differentiation of Th1 and Th17 pathway), increases the release of IL-10 (cytokines with broad anti-inflammatory activity) and increases release of chemokines MIP-3a/CCLL22 (chemokines which play a role in the recruitment of CCR4 expressing Treg). In addition, by modulating DC-derived cytokines, 1,25(OH)2D influences the balance of Th-derived cytokines by inhibiting the production of inflammatory Th1 (IFN-γ, IL-2) and Th17 (IL-17 and IL21) pathways and promotes the Th2 phenotype.32
With respect to tolerance and the autoimmune process, 1,25(OH)2D can enhance Treg cells and promotes tolerogenic DC and then influences the tolerance process in transplantation and prevents the development of autoimmune diseases.13 Jeffery et al., investigated the role of 1,25(OH)2D on Treg cells and proinflammatory cytokines using healthy donor blood cultures. They showed that stimulation of 1,25(OH)2D on Treg inhibits the production of several cytokines such as IFN-γ, IL-21 and IL-17. Also, 1,25(OH)2D stimulates the expression of FoxP3 and CTLA-4 on Treg cells with the help of IL-2. The addition of IL-2 alone in the culture medium did not significantly increase the expression of Foxp3. But, if the addition of IL-2 is accompanied by 1,25(OH)2D, there was a sharp increase (two fold) in the expression of Foxp3 and CTLA-4 in Treg cells. It showed that 1,25(OH)2D and IL-2 have a synergistic effect on activated T cells, serves as a powerful anti-inflammatory, and can stimulate physiological adaptive Treg cells.36
Boonstra did an experimental animal study using naive CD4 Mel14 T cells with plate bound antiCD3 and soluble anti-CD28 mAb in the presence or absence of vitamin D3. The cells were repeatedly stimulated, and their cytokines developed into a highly polarized Th2 population when cultured with vitamin D3, similarly to cultures with APC. Although APC is the immunomodulatory target of vitamin D3, vitamin D3 can also directly stimulate the TCR, influence the polarization of naive T cells and then inhibit Th1 pathway (IFN-γ production) and increased Th2 pathway (IL-4, IL-5 and IL-10). This is the first study that shows the direct effect of vitamin D3 on Th cells in the absence of APC.37
Mahon showed that the targets of vitamin D depends on the differentiation and activation status of T-cells. Since naive T cells have little VDR, they are relatively unresponsive to vitamin D 1,25(OH)2 in the first 24 hours of activation.38
Although T cell activation will stimulate B cells, 1,25(OH)2D can directly affect B cells through the inhibition of the proliferation and differentiation of plasma cells, the inhibition of Ig secretion, the improvement of B cell memory and the stimulation of B cell apoptosis. A study by Heine using healthy donor blood cultures showed that B cells can regulate immune response by producing 1,25 (OH)2D through an autocrine mechanism as well as increase the expression of IL-10 by B cells alone, T cells and DC. IL-10 is a pleiotropic cytokine that inhibits antigen presentation by DCs and macrophages and also inhibits T cell activation. In addition, IL-10 inhibits the migration of B cells and stimulates them to change into plasmablasts.32,39
Click here to download Figure 2
Figure 2. The role of vitamin D 1,25(OH)2 in innate and adaptive immune response32
Adapted from Baeke F. Current Opinion in Pharmacology 2010

Vitamin D 1,25(OH)2 influence innate immune cells and adaptive immune cells. In innate immune cells, vitamin D would improve the ability of phagocytosis and chemotaxis and decrease the expression of TLR-2 and TLR-4. Vitamin D also prevents the excessive production of proinflammatory cytokines. To the adaptive immune cells, vitamin D is able to decrease the expression of costimulatory molecules, MHC-II and the cytokine IL-12, as well as improve the function of T reg cells by increasing cytokine IL-10. Vitamin D also has a direct effect on CD4 T cells to shift the immune response to the Th2 pathway and Treg.
Although immune cells have VDR and enzyme 1α-OH-ase, the role of vitamin D in autoimmune disease is still controversial.13,40 Epidemiologic study demonstrated the effect of vitamin D deficiency on the incidence of multiple sclerosis (MS), diabetes mellitus type 1 (DM1), inflammatory bowel disease (IBD), rheumatoid arthritis (RA) and systemic lupus erythematosus (SLE).27,31
In observational ecologic studies, some autoimmune diseases such as IBD, MS, RA are more common in the northern latitudes which have low sunlight exposure. Besides that, MS has a correlation with winter births (maternal vitamin D status during pregnancy is low) and disease recurrence is more frequent in winter.23,40-41
Prospective studies showed that 25(OH)D levels are associated with RA incidence, DM1 and MS. It is not clear whether a low concentration of vitamin D is the result of autoimmune disease or is a cause of autoimmune disease.41 A systematic review reported the association between vitamin D and the risk of autoimmune disease and effects of vitamin D supplementation on their clinical presentation. The study showed vitamin D could prevent the incidence of DM1. Physiopathologic studies show that among individuals who have a genetic risk factor, hypovitaminosis D can impair tolerance.41-43
The role of oral supplementation of vitamin D in autoimmune disease was also reported in SLE, RA and IBD.44 Studies in Malang, Indonesia, showed the serum vitamin D 25(OH) level of SLE patients were significantly lower than that of healthy subjects (22.80 ± 16:23 ng/dl vs. 35.15 ± 7.61 ng/dl, p = 0:00). As many as 55.5% of SLE patients have vitamin D deficiency and the levels are inversely correlated with antibody level (anti-ds-DNA and anti-vitamin D).45 A systematic review showed that vitamin D can improve several sign and symptoms of the disease, but is not necessarily related to the severity of the disease.42
The role of vitamin D on DC profile in autoimmune diseases has not been widely reported. Handono reported a negative correlation between vitamin D 25(OH) level with the expression of DC maturation markers (CD11c +, CD40, CD83 and CD86) in SLE.35
In Crohn's disease, the intervention of vitamin D 25(OH) or vitamin D 1,25(OH)2 in monocyte-derived dendritic cells culture (MDDC) impairs the maturation of DC, which is characterized by decreased expression of HLA-DR molecules, CD80, CD83, CD86 and increased expression of CD14. In addition to this, intervention of vitamin D in vitro also decreased the production of proinflammatory cytokines such as tumor necrosis factor-α (TNF-α), IL-12 and IL-10 but elevates IL-6.46 Aside from the vitro study, Bartels also gave vitamin D,1200 IU/day of vitamin D3 for six months to Crohn's disease patients. Before and after administration of oral vitamin D3, he analyzed DC maturation and cytokine production. Oral administration of vitamin D impairs the maturation of DC shown by decreasing expression of CD80 molecules.19 Besides impairing the expression of CD80, the concentration of inflammatory cytokines, TNF-α and IFN-γ, also decline.19,46 Vitamin D intervention on DC maturation has also been reported in SLE patients, it decreased the expression of HLA-DR, CD40 and CD86.47
Graves' disease is characterized by excessive autoantibodies that bind to the TSHR in thyroid cells and stimulates thyroid hormone production resulting in hyperthyroidism.5,48 It is the most common cause of hyperthyroidism (60-80%), affecting mainly women aged 40 to 60 years. The pathogenesis is still not fully understood but genetic predisposition and environmental factors leads to disruption tolerance to self-antigens and triggers an autoimmune thyroid disease AITD.49
Under normal conditions, the immune response will protect the body from foreign proteins and is able to tolerate self-antigens through central and peripheral tolerance. The first defense mechanism against autoimmunity is that not every Ag is able to stimulate T cells, but only Ag presented by specific MHC molecules.
Central tolerance occurs in the thymus gland, if the body eliminates autoreactive T cells and T cells that interact with immature DC. However, not all T cells will be destroyed in the thymus, some will escape into the peripheral circulation.
In peripheral tolerance, at steady state (prior to infection and inflammation), DCs are in the immature state and will be able to recognize self-antigens or foreign weak Ag (protein) and create tolerance to the Ag. This stage is important because the DC should be able to distinguish between self-antigens and foreign potent Ag. When there is an infection or inflammation, the environment around the DC changes, with an increase of proinflammatory cytokines that stimulates the maturation of DC. Once mature DC develop, the potential self antigens can potentially trigger adaptive immune responses against self antigens (autoimmunity). However, if the tolerance to self-antigens have occurred prior to the infection, the immune response to self-antigens can be prevented. The failure of tolerance to self-antigens will induce autoimmune diseases.50-51
DC maturation plays an important role in the process of tolerance and autoimmunity by changing the nature of the iDC, which has ability to capture antigens but are not immunogenic, into DC which have weak endocytic ability but are immunogenic [DCs are able to stimulate effector T cells (CD4, CD8) and raise the production of cytokines that supports a subsequent immune response (IFN-γ)].51 Beside inducing tolerance, in vitro studies showed that repeated stimulation of T cells with iDC will develop a population of T cell that produce IL-10 (Treg cells). Based on the description above, the iDC plays an important role in central and peripheral tolerance.52-53
The initial immune response in GD requires both a specific and non-specific signal. Ag-specific signals are exogenous or endogenous, whereas the non-specific signals are costimulatory signals and inflammatory cytokines. Endogenous sources can be the dead-thyrocyte, whereas exogenous signals can be either viral or bacterial. The TSH receptor undergoes a posttranslational split, forming 2 subunit structures with subunit A serving as a self antigen. The adaptive immune response in GD starts with the presentation of this specific Ag signal (exogenous or endogenous) by DCs, to T cells.48,54 In the early stages, T cell receptors (TCR) on the surface of T cells interacts with MHC-peptide complexes on the APC. After this stage, naive T cells require subsequent signals to activate processes.
The next stage is the interaction between CD28 on the T cell surface and costimulatory molecules CD80/CD86 on the APC. The existence of two signals (TCR with MHC-peptide and CD28 with CD 80/CD86) will activate naive T cells and subsequent immune response that lead to the formation of antibodies by B cells. 55
In addition to potent APC, the autoimmune process is also triggered by environmental factors, including immune cell-produced by cytokines due to Ag stimulation. The cytokines (IL-1β, TNF-α, IL-12, IFN-γ, IFN-α, IL-13, etc.) will increase the efficiency of the antigen presentation by increasing the expression of MHC and costimulatory molecules on APC. If there are no inflammatory cytokines, the costimulatory signal appears weak or absent, then the antigen presentation is suboptimal and induces the tolerance process.48
Kabel et al., compared the profile of tDC in GD and in normal thyroid glands. The study involved thyroid tissues of 13 GD patients who underwent surgery and 9 normal thyroid tissue (6 specimens were obtained at laryngectomy (laryngeal carcinoma and 3 specimens were obtained from autopsy). They were able to show normal human thyroid glands contain fewer population of mononuclear cell (MNC) which reacted strongly positive for class II MHC markers, similar with the morphologic characteristic of DC. This MNC did not react to RFD1 and L25 markers (markers characteristic of DC on secondary lymph organs which are actively involved in immune response). These findings suggest that there is a small number of DC in normal thyroid gland but they are not actively involved in the immune response and in antigen handling. The tDC serve to remove foreign objects and materials that have been damaged by the thyroid to the thyroid-draining lymph nodes. In thyroid glands of GD, there are more tDC and they react more positively to markers RFD1 and L25, which were shown to be actively involved in the immune response including autoantigen handling. Some tDC are even seen to interact with lymphocytes in the thyroid gland. The results of this study correlated with a previous animal study on laboratory rats, were the number of tDC was increased and it preceded the appearance of anticolloid antibodies in the circulation.11 The DC are found outside the thyroid gland, in draining lymph nodes around the thyroid and lymph vessels. A series of general immunological processes follows: tDC captures antigen (as immature DC) and brings the antigen to the lymph nodes for presentation to T cells (transform to mature DC). The migration process of mature DC is followed by the expansion and maturation of T cells and B cells in the lymph channels, even infiltrating the thyroid gland.48
Fourteen years later, Quadbeck examined several types of DC in Graves’ thyroid glands. Quadbeck’s study involved thyroid tissues of 15 GD patients who underwent thyroid surgery. The results showed that there were three populations of t-DC: 1) Immature t-DC (MHC II+/CD40-/CD80-) found in perifollicular thyroid tissue, 2) Partially mature CD80+ t-DC located in connective tissue and interstitial clusters, and 3) Mature t-DC (MHC II+/ CD40+/CD80+/RFD1+) were in clusters and adjacent/interacting with activated Th cells (CD4+/MHC-II+). Immature t-DC were also found in venous capillaries. The number of immature t-DC in GD patients were higher than that of healthy subjects and toxic adenoma (toxic goiter, TG), respectively: 95%; 55%; and 51%. It means that the t-DC in some GD patients undergo maturation in clusters (groups) which resemble lymphoid tissue. The maturation of t-DC in these clusters is also supported by the intimate contact between t-DC and CD4+/MHC class II+ Th cells. The expression of HLA-DR by thyrocytes were elevated, which might indicate the cooperation between DC, macrophages and thyrocytes in triggering autoimmunity in GD.10
The role of DC and the association between DC and Treg in GD was investigated by Mao et al., involving 77 untreated Graves' disease (uGD) 13 euthyroid patients with ATD (euthyroid graves' disease, eGD), 15 patients with euthyroid Hashimoto's thyroiditis (euthyroid Hashimoto, eHT) and 74 healthy controls. The study showed that the proportion of Treg cells in the uGD is lower (1.57±0.67% vs 3.36±0.96%, p<0.001) than that of the healthy controls, but not in the eGD group (2.98±0,88%) and eHT (3.09±1.02%). Although the number was lower, the function of T-reg cells in uGD was maintained. Moreover, the proportion of T-reg cells was also negatively correlated with TSHR antibody level in the uGD (r=-0.735, p<0.001). The proportion of costimulatory molecules CD86, CD80 and CD40 increased in the uGD when compared with the control group. The proportion of pDC in the uGD was significantly higher than that of control group (41.95±8.39% vs 32.85±8.02%, p<0.001), eGD group (31.63±7.07%, p<0.001) and HT group (28.50±3.96%, p<0.0001). Furthermore, the pDC/DC ratio in the uGD was the highest and the ratio of pDC/DC is negatively related to the proportion of T-reg cells (r=-0.689, p<0.001). This phenomenon showed that there was polarization of pDC among uGD that might play a role in reducing the proportion of T-reg cells. Since plasmacytoid DC (pDC) is a major producer of IFN-α, the increased proportion of pDC will raise the cytokines IFN-α in the uGD. The T-reg cells undergoing apoptosis in the uGD with IFN-α dependent mechanism.
GD patients have predominant Th2 cytokines and increased Th17 cells. The Th2 pathway will secrete IL-4 that will activate B cell-producing antibodies. 54 The imbalance of Th1 and Th2 pathways in GD patients is demonstrated by several studies that investigated the Th1 and Th2 cells derived cytokines, either in the thyroid gland or peripheral blood vessels. To see the differences of intrathyroidal cytokine mRNA production between autoimmune and non-autoimmune thyroid disorders, Heuer et al., analyzed cytokine mRNA expression levels in thyroid tissue samples from 13 GD subjects, 2 HT subjects, 5 multinodular nontoxic goiter (MNNT) subjects and 4 TA subjects. The GD patients are divided into two groups based on the titer of antibody as follows: anti-thyroperoxidase (anti-TPO) > 4000 U/mL called GD high and anti-TPO < 200 U/mL called GD low. The results showed that the GD high group had higher IL-4 mRNA concentration as much 2-4 times more than that of among GD low group. Furthermore, the concentration of IL-10 mRNA among GD high group were significantly higher than that of other groups. The concentrations of IFN-γ, IL-1/3, IL-8 and CD25 mRNA among GD high group were also significantly higher than those in the GD low group. The highest level of IFN-γ, IL-2 and CD25 mRNA were found among the HT group, while the lowest level of mRNA were found among the TA group. The study showed that among GD high group, there was a shift to Th2 pathway, whereas among HT group the Th1 pathway was pronounced which indicate the dominant T cell mediated cytotoxic process.6
Phenekos et al., documented the pattern of immune response in GD by measuring both Th1 and Th2 serum cytokine profiles among patients suffering from autoimmune disease and toxic nodular goiter. The study involved 25 normal subjects (control) and 43 patients. Twenty-five patients suffered from GD, 21 from HT and 7 from toxic nodular goiter. Measured serum cytokines by enzyme-linked immunosorbent assay (ELISA) included: IL-2, IL-1β, IFN-α, TNF-β, IL-12, IL-5, IL-10, IL-8, IL-4 and IL-5. The study showed that GD subjects had a higher level of IL-4 and IL-5 than those of the other groups, while the HT subjects had a higher level of IL-2, IFN-γ, IL-12 and IL-18 than those of the other groups. It means that GD subjects had dominant Th2 cytokine pathway, whereas HT subjects had dominant Th1 cytokine pathway.9
The role of Th1/Th2 pathway mechanism in the pathogenensis of GD was also examined in experimental animals by Nagayama et al. Hyperthyroidism is induced by using an adenovirus that expresses human TSHR A subunit. The study showed that the disruption of IFN-γ and IL-4 in BALB/c mice will prevent hyperthyroidism.7
In addition to the proportion of Th1 and Th2 cytokine profile, Nanba et al., reported that there was an increase of Th17 cells expression among GD patients which has proinflammatory activity. The study involved 17 patients with Hashimoto's thyroiditis (Hashimoto thyroiditis, HT) who received thyroxine (severe HT), 17 patients with Hashimoto 's thyroiditis without thyroxine (mild HT), 18 euthyroid GD with anti-thyrotropin receptor antibody (TRAb) positive after 5 years of anti-thyroid drug therapy (intractable GD), 17 euthyroid GD patients with negative TRAb for more than 2 years after stopping anti-thyroid drug treatment (GD in remission) and 10 control subjects. The study reported that the proportion of Th2 cells among the intractable GD group and mild HT were significantly higher than that of the control group. The ratio of Th1/Th2 cells in severe HT was higher than that of mild HT. The proportion of Th17 cells in patients with intractable GD and GD remission were higher than that of control group. Furthermore, the proportion of Th17 cells in intractable GD group was higher than that of GD with remission.8
The relationship between vitamin D status with GD is not widely reported. From the point of view of genetic studies, a meta-analysis reported an association between VDR polymorphisms with GD in Asian populations, but not in the Caucasian population.57
The Yamashita study in Japan involving 208 GD patients showed that the prevalence of vitamin D deficiency among female GD reached 39.7%, it was higher than that of males, by as much as 17.8%. This prevalence is influenced by seasonal change, the highest prevalence is 60%, during April to June; the lowest prevalence is less than 20%, during July-September. The mean level of vitamin D in men and women GD subjects men and women respectively were 41.3±15.0 and 31.8±13.3 nmol/l. This value is lower than that of a previous study by Kobayashi, who examined 758 healthy subjects in Japan whose mean concentration of 25(OH)D was 59.4 nmol/l (~23.76 ng/ml). Females aged 20-29 years had the lowest vitamin D level and the highest prevalence of vitamin D deficiency. Low levels of vitamin D among young women can be triggered by avoiding sun exposure and the use of sunscreen; men tend to spend more time engaged in outdoor activities.20,43
Eleven years later, after Yamashita's study, Yasuda conducted a study in the same place among 26 female naive GD and 46 healthy female subjects, to investigate the effect of vitamin D level on the volume of thyroid gland. They found the mean level of vitamin D among GD was significantly lower than that of the control group (14.4±4.9 vs 17.1±4.1 ng/ml, p<0,05). Vitamin D level is associated with thyroid volume (r=0.45, p<0.05) but not with thyroid function and the TRAb level. Yasuda also investigated the difference in vitamin D level between remission GD and non-remission GD. The study involved 18 remission GD, 36 non-remission GD and 49 control subjects. Vitamin D level among non-remission GD is significantly lower than that of remission GD and the control group (14.5±2.9 vs 18.2±5.1 vs 18.6±5.3 ng/ml). Among GD, vitamin D level does not change due to treatment with ATD. 41-42
Cross sectional studies showed there is an association between vitamin D level and GD, including the remission status, however, there is no large prospective study that investigates the effects of vitamin D status on the onset and clinical remission of GD.21,58 In animal studies, Misharin investigated the relationship between vitamin D deficiency and hyperthyroidism induced in BALB/c mice. The study demonstrated that an environmental factor, vitamin D, has only minor effects on induced immunity to the TSHR but that it directly affects thyroid function in mice.59
Click here to download Figure 3
Figure 3. The estimation diagram about the immuno-modulatory effects of vitamin D in GD. Legend: TSHR (Thyroid Stimulating Hormone Receptor); T4 (Thyroxin); T3 (Triiodothyronin); DC (Dendritic Cell); MHC (Major Histocompatibility Complex); LPS (Lipopolysaccharide); APC (Antigen Presenting Cells); TCR (T Cell Receptor)

The role of vitamin D supplementation on clinical improvement of untreated hyperthyroid patients was reported by Tani in Japan. The 24-week clinical trial involves 30 patients, and aims to study the effects of vitamin D 1,25(OH)2 on the decline of thyroid hormone levels in 30 hyperthyroid patients. The subjects were randomly divided into two groups, the first group received 30 mg of MMI, while the second group receive the same dose of MMI with added vitamin D 1,25(OH)2. During the monitoring period, the total doses of MMI did not differ between the two groups. The levels of FT3, fT4, T3 and T4 decreased significantly and more rapidly in the second group, as well as did the increase in TSH level. The level of TRAb did not differ between the two groups. The study showed the benefits of vitamin D 1,25 (OH)2 supplementation in GD patients but the mechanism was still unknown at that time. However, in experimental animal studies in the same year on the effect of vitamin D on thyrocytes, it may be that he beneficial effects of vitamin D in hyperthyroid patients may be due to a direct effect on the thyrocytes. It is not known whether the clinical improvement is associated with the role of vitamin D in adaptive immune response in GD. It should be explored whether vitamin D influences the maturation of DCs and the activation of T cell or B cell.60
Theoretically, the effects of vitamin D as an immunomodulator influences the tolerance process which consists of the maturation of DC and induction of Treg cell. Impaired immune response in GD may include the activity of APC (DC), the polarization of Th1/Th2 and downregulation of Treg cells. However, whether vitamin D system affects the natural history of GD, still needs to be proven.
Adaptive immune response in GD starts with the stimulation of mature DC with self antigen (TSHR peptide) and activation T helper cell. T helper cell will stimulate B cell to produce autoantibody. Vitamin D may alter the series of immune process in GD through several ways. First, vitamin D will reduce the maturation of DC; second, vitamin D will directly suppressed the Th cell activation; third, vitamin D will hamper the production of autoantibody by the B cell and the fourth, vitamin D will stimulate Treg cell to suppress the activation of Th cells and B cells.
1. Weetman AP. Graves' disease. N Engl J Med. 2000;343(17):1236-48.
2. Weetman AP, McGregor AM, Hall R. Evidence for an effect of antithyroid drugs on the natural history of Graves' disease. Clin Endocrinol (Oxf). 1984;21:163-72.
3. Cooper DS. Hyperthyroidism. The Lancet. 2003;362:459-68.
4. Orgiazzi J. Thyroid autoimmunity. Presse Med. 2012;41:e611-25.
5. Swain M, Swain T, Mohanty BK. Autoimmune thyroid disorders-An update. Indian Journal of Clinical Biochemistry. 2005;21(1):9-17.
6. Heuer M, Aust G, Ode-Hakim S, Scherbaum WA. Different cytokine mRNA profiles in Graves' disease, Hashimoto's thyroiditis, and nonautoimmune thyroid disorders determined by quantitative reverse transcriptase polymerase chain reaction (RT-PCR). Thyroid. 1996;6(2):97-106.
7. Nagayama Y, Saitoh O, Mclachlan SM, Rapoport B, Kano H, Kumazawa Y. TSH receptor-adenovirus-induced Graves' hyperthyroidism is attenuated in both interferon-γ and interleukin-4 knockout mice; implication for the Th1/Th2 paradigm. Clin Exp Immunol. 2004;138:417-22.
8. Nanba T, Watanabe M, Inoue N, Iwatani Y. Increases of the Th1/Th2 cell ratio in severe Hashimoto's disease and in the proportion of Th17 cells in intractable Graves' disease. Thyroid. 2009;19(6):495-.
9. Phenekos C, Vryonidou A, Gritzapis AD, Baxevanis CN, Goula M, Papamichail M. Th1 and Th2 serum cytokine profiles characterize patients with Hashimoto's thyroiditis (Th1) and Graves' disease (Th2). Neuroimmunomodulation. 2004;11:209-13.
10. Quadbeck B, Eckstein AK, Tews S, Walz M, Hoermann R, Mann K, et al. Maturation of thyroidal dendritic cells in Graves' Disease. Scand J Immunol. 2002;55:612-20.
11. Kabel PJ, Voorbij HAM, Haan MD, Gaag RDVD, Drexhage HA. Intrathyroidal dendritic cells. J Clin Endocrinol Metab. 1988;65:199-207.
12. Adams JS, Liu PT, Chun R, Modlin RL, Hewison M. Vitamin D in the defense of the human immune response. Ann NYAcadSci. 2007;1117:94-105.
13. Adorini L, Penna G. Control of autoimmune diseases by the vitamin D endocrine system. Nature Clinical Practice Rheumatology. 2008;4(8):404-12.
14. Aranow C. Vitamin D and the immune system. J Investig Med. 2011;59(6):881-6.
15. Brown AJ, Dusso A, Slatopolsky E. Vitamin D. Am J Physiol Renal Physiol. 1999;277:157-75.
16. DeLuca H. Overview of general physiologic features and functions of vitamin D. Am J Clin Nutr. 2004;80:1689s-96s.
17. Etten Ev, Mathieu C. Immunoregulation by 1,25-dihydroxyvitamin D3: Basic concepts. Journal of Steroid Biochemistry & Molecular Biology. 2005;97:93-101.
18. Cantorna MT, Mahon BD. Mounting evidence for vitamin D as an environmental factor affecting autoimmune disease prevalence. Experimental Biology and Medicine. 2004;229:1136-42.
19. Bartels LE, Bendix M, Hvas CL, Jorgensen SP, Agnholt J, Agger R, et al. Oral vitamin D3 supplementation reduces monocyte-derived dendritic cell maturation and cytokine production in Crohn's disease patients. Inflammopharmacol. 2013.
20. Yamashita H, Noguchi S, KeisukeTakatsu, Koike E, Murakami T, Watanabe S, et al. High prevalence of vitamin D deficiency in Japanese female patients with Graves' disease. Endocrine Journal. 2001;48(1):63-9.
21. Yasuda T, Okamoto Y, Hamada N, Miyashita K, Takahara M, Sakamoto F, et al. Serum vitamin D levels are decreased in patients without remission of Graves' disease. Endocrine. 2013;43:230-2.
22. Yasuda T, Okamoto Y, Hamada N, Miyashita K, Takahara M, Sakamoto F, et al. Serum vitamin D levels are decreased and associated with thyroid volume in female patients with newly onset Graves' disease. Endocrine. 2012;42:739-41.
23. Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357:266-81.
24. Dusso AS, Brown AJ, 2005;289:8-28. SEAJPRP. Vitamin D. Am J Physiol Renal Physiol. 2005;289:8-28.
25. Kulie T, Groff A, Redmer J, Hounshell J, S S. Vitamin D: An evidence-based review. J Am Board Fam Med. 2009:698-706.
26. Zittermann A. Vitamin D in preventive medicine: Are we ignoring the evidence? British Journal of Nutrition. 2003;89:552-72.
27. Li J, Byrne ME, Chang E, Jiang Y, Donkin SS, Buhman KK ea-. 1, 25-dihidroxyvitamin D hidroxylase in adipocytes. J Steroid BiochemMol Biol. 2008;112:122-6.
28. Ogunkolade BW, Boucher BJ, Prahl JM, Bustin SA, Burrin JM, Noonan K ea-. Vitamin D receptor (VDR) mRNA and VDR protein levels in relation to vitamin D status, insulin secretory capacity and VDR genotype in Bangladeshi Asians. . Diabetes. 2002;51:2294-312.
29. Yetley E. Assessing the vitamin D status of the US population. Am J ClinNutr 2008;88:558S-64S.
30. Hagenau T, Vest R, Gissel TN, Poulsen CS, Erlandsen M, Mosekilde L, et al. Global vitamin D levels in relation to age, gender, skin pigmentation and latitude: An ecologic meta-regression analysis. Osteoporos Int. 2009;20:133-40.
31. Mithal A, Wahl DA, Bonjour JP, Burckhardt P, Dawson-Hughes B, Eisman JA, et al. Global vitamin D status and determinants of hypovitaminosis D. Osteoporosis Int. 2009;20:1807-20.
32. Baeke F, Takiishi T, Korf H, Gysemans C, Mathieu C. Vitamin D: Modulator of the immune system. Current Opinion in Pharmacology. 2010;10:482-96.
33. Berer A, Stockl J, Majdic O, Wagner T, Kollars M, Lechner K, et al. 1,25-dihydroxyvitamin D3 inhibits dendritic cell differentiation and maturation in vitro. Experimental Hematology. 2000;28:575-83.
34. Piemonti L, Monti P, Marina S, Fraticelli P, Leone BE, Cin ED, et al. Vitamin D3 affects differentiation, maturation and function of human monocyte-derived dendritic cells. Journal of Immunology. 2000;164:4443-51.
35. Handono K, Daramatasia W, Pratiwi, Sunarti S, Wahono S, Kalim H. Low level of vitamin D increased dendritic cell maturation and expression of interferon-γ and interleukin-4 in systemic lupus erythematosus. Journal of pharmacy and biological sciences. 2012;2(4):37-43.
36. Jeffery LE, Burke F, Mura M, Zheng Y, Qureshi OS, al. HMe. 1,25-dihydroxyvitamin D3 and interleukin-2 combine to inhibit T cell production of inflammatory cytokines and promote development of regulatory T cells expressing CTLA-4 and FozP3. J Immunol 2009;183:5458-67.
37. Boonstra A, Barrat FJ, Crain C, Heath VL, Savelkoul HFJ, O'Garra A. 1α,25-dihydroxyvitamin D3 has a direct effect on naive CD4+ to enhance the development of Th2 cells. The Journal of Immunology. 2001;167:4974-80.
38. Mahon BD, Wittke A, Weaver V, Cantorna MT. The targets of vitamin D depend on the differentiation and activation status of CD4+ T cells. J Cell Biochem. 2003;89:922-32.
39. Heine G, Niesner U, Chang H-D, Steinmeyer A, Zugel U, Zuberbier T, et al. 1,25-dihydroxyvitamin D3 promotes IL-10 production in human B cells. Eur J Immunol. 2008;38:2210-8.
40. Schwalfenberg GK. Solar radiation and vitamin D: Mitigating environmental factors in autoimmune disease. Journal of Environmental and Public Health. 2012:1-9.
41. Kriegel MA, Manson JE, Costenbader KH. Does vitamin D affect risk of developing autoimmune disease?: A sytematic review. Semin Arthritis Rheum. 2011;40:512-31.
42. Antico A, Tampoia M, Tozzoli R, Bizzaro N. Can supplementation with vitamin D reduce the risk or modify the course of autoimmune disease? A systematic review of the literature. Autoimmune Rev. 2012;12(2):127-36.
43. Holick MF. Sunlight and vitamin D for bone health and prevention ofautoimmune disease, cancers and cardiovascular disease. Am J Clin Nutr 2004;80:1678s-88s.
44. Leventis P, Patel S. Clinical aspects of vitamin D in the management of rheumatoid arthritis. Rheumatology. 2008;47:1617-21.
45. Handono K, Gani AA, Ekawati M, Wahono S. Serum level of vitamin D and autoantibodies level in systemic lupus erythematosus (SLE) patients. Journal of Pharmacy and Biological Sciences. 2012;3(4):16-20.
46. Bartels LE, Jogersen SP, Bendix M, Hvas CL, Agnholt J, Agger R, et al. 25-Hydroxy vitamin D3 modulates dendritic cell phenotype and function in Crohn’s disease. Inflammopharmacol. 2013;21:177-86.
47. Ben-Zvi I, Aranow C, Mackay M, Stanevsky A, Kamen DL, Marinescu LM, et al. The impact of vitamin D on dendritic cell function in patients with systemic lupus erythematosus. Plos ONE. 2010;5(2):1-8.
48. Vasu C, Holterman MJ, Prabhakar B. Modulation of dendritic cell function and cytokine production to prevent thyroid autoimmunity. Autoimmunity. 2003;36(6-7):389-96.
49. Kawashima A, Tanigawa K, Akama T, Yoshihara A, Ishii N, Suzuki K. Innate immune activation and thyroid autoimmunity. J Clin Endocrinol Metab. 2011;96:3661-71.
50. Tsokos GC, Goust J-M, Virella G. Tolerance and autoimmunity. In: Virella G, editor. Medical Immunology. 5th ed. New York: Marcel Dekker; 2001. p. 313-40.
51. Steinman RM, Hawiger D, Liu K, Bonifaz L, Bonnyay D, Mahnke K, et al. Dendritic cell function in vivo during the steady state: A role in peripheral tolerance. Ann N Y Acad Sci. 2003;987:15-25.
52. Moser M. Antigen presentation, dendritic cells and autoimmunity In: Rose NR, Mackay IR, editors. The Autoimmune Disease. 4th ed. UK: Elsevier Academic Press; 2006. p. 37-46.
53. Shortman K, Liu Y-J. Mouse and human dendritic cell subtypes. Nature Reviews. 2002;2:151-61.
54. Prabhakar BS, Bahn RS, Smith TJ. Current perspective on the pathogenesis of Graves’ disease and ophthalmopathy. Endocrine Reviews 2003;24(6):802-35.
55. Weetman AP, DeGroot LJ. Autoimmunity to the thyroid gland. Thyroid Manager. 2013:1-127.
56. Mao C, Wang S, Xiao Y, Xu J, Jiang Q, Jin M, et al. Impairment of regulatory capacity of CD4+CD25+ regulatory T cells mediated by dendritic cell polarization and hyperthyroidism in Graves' disease. The Journal of Immunology. 2011;186:4734-43.
57. Zhou H, Xu C, Gu M. Vitamin D receptor (VDR) gene polymorphisms and Graves' disease: A meta-analysis. Clin Endocrinol (Oxf). 2009;70(6):938-45.
58. Pantazi H, PD P. Changes in parameters of bone and mineral metabolism during therapy for hyperthyroidism. J Clin Endocrinol Metab. 2000;85:1099-106.
59. Misharin A, Hewison M, Chen C-R, Lagishetty V, Aliesky HA, Mizuturi Y, et al. Vitamin D deficiency modulates Graves' hyperthyroidism induced in BALB/c mice by thyrotropin receptor immunization. Endocrinology. 2009;150(2):1051-60.
60. Kawakami-Tani T, EtsushiFukawa, Tanaka H, Abe Y, Makino I. Effect of lα-Hydroxyvitamin D3 on serum levels of thyroid bormones in hyperthyroid patients with untreated Graves' Disease. Metabolism. 1997:1184-118.
Articles and any other material published in the JAFES represent the work of the author(s) and should not be construed to reflect the opinions of the Editors or the Publisher.
Authors are required to accomplish, sign and submit scanned copies of the JAFES Declaration that the article represents original material that is not being considered for publication or has not been published or accepted for publication elsewhere.
Consent forms, as appropriate, have been secured for the publication of information about patients; otherwise, authors declared that all means have been exhausted for securing such consent.
The authors have signed disclosures that there are no financial or other relationships that might lead to a conflict of interest. All authors are required to submit Authorship Certifications that the manuscript has been read and approved by all authors, and that the requirements for authorship have been met by each author.