The Metabolic Syndrome (MetS) represents a combination of cardio-metabolic risk factor determinants including central adiposity, insulin resistance, glucose intolerance, dyslipidemia, non-alcoholic fatty liver disease (NAFLD) and hypertension. It is rapidly increasing in prevalence worldwide as a consequence of the obesity epidemic. As a result, MetS will have a considerable impact on the global incidence of cardiovascular disease and type 2 diabetes (T2DM).1 Insulin resistance is thought to be the primary underlying abnormality leading to MetS.2
Fetuin-A (also known as human protein alpha-2-Heremans-Schmid-glycoprotein, AHSG) and other circulating proteins have been shown to be involved in the regulation of insulin sensitivity. Fetuin-A is a liver-synthesized protein that is secreted into serum. It can bind the insulin receptor and inhibit insulin signaling in skeletal muscle and hepatocytes, inhibiting insulin signal transduction and resulting in insulin resistance in the target tissues.3 In humans, higher levels of fetuin-A are associated with higher TG, low-density lipoprotein cholesterol (LDL-C), BMI, and insulin resistance. 4 Higher fetuin-A concentrations were associated with accumulation of visceral adipose tissue, a major component of the MetS.5 The link between fetuin-A, obesity, insulin resistance, NAFLD, and MetS in humans is less clear. Some studies in adults have reported significant associations between fetuin-A, NAFLD and insulin resistance.6 Most of these studies were cross-sectional and limited by many confounders. Longitudinal studies are preferable to clarify these metabolic relationships.
Lifestyle modifications to address overweight, physical inactivity and an atherogenic diet have been recommended as a foundation for the management of MetS.7 However, lifestyle modification alone is often unable to achieve clinically meaningful weight loss.8
Metformin, a biguanide oral antidiabetic agent, has been shown to reduce weight, hyperinsulinemia and hyperglycemia in adult patients with T2D. It is recommended as first-line pharmacotherapy in overweight and obese T2D patients.9 While metformin has been found to attenuate the insulin-sensitizing effect of exercise, it has been found to have beneficial effects on inhibition of platelet aggregation, antioxidant activity, weight reduction, lipid parameters (total cholesterol, HDL-C, LDL-C and TG) and arterial hypertension.10-12 Metformin can be given safely to euglycemic patients, as it does not induce hypoglycemia.13 Furthermore, in ob/ob mice, a model of hepatic steatosis, metformin reversed hepatomegaly, hepatic fat accumulation and ALT abnormalities by reducing hepatic tumor necrosis factor-α (TNF-α) expression. 14
The aim of this study was to assess the effect of lifestyle modification on cardio-metabolic risk factors and fetuin-A levels with or without metformin in relation to improvement of insulin sensitivity in patients with the MetS.
Study subjects who met the 2006 IDF definition of the metabolic syndrome were recruited from the H. Adam Malik Hospital in Medan, Indonesia. The criteria included central obesity (WC of ≥ 90 cm in men and ≥ 80 cm in women of Asian ethnicity) plus any 2 of the following 4 factors: elevated triglycerides (≥ 150 mg/dL) or specific treatment for this lipid abnormality, reduced HDL-C (< 40 mg/dL in men and < 50 mg/dL in women) or specific treatment for this lipid abnormality, elevated BP blood pressure (SBP ≥130 mmHg or DBP ≥ 85 mmHg) or treatment of previously diagnosed hypertension, and elevated FPG (≥ 100 mg/dL) or previously diagnosed type 2 diabetes.15,16 Exclusion criteria included smoking, known cardiovascular disease or any major illness, and use of medication that could affect laboratory test results. Forty subjects gave their full informed consent to participate and undergo lifestyle modification for 12 weeks. They were assigned randomly to treatment with either placebo or metformin (Figure 1). Each participant was advised to take one capsule three times a day after meal. For the placebo group, the capsule contained calcium gluconate 500 mg. For the metformin group, the capsule contained metformin 500 mg. No vitamins or other nutritional supplements were prescribed. Prior to initiation and during the study, all the participants discussed lifestyle modification including diet and physical activity with a trained health nurse. To facilitate behavior change, each participant received an instructional leaflet and a diary to record behavioral performance, diet, physical activity, WC and weight. Every week, all participants attended a follow-up meeting for confirmation of compliance and monitoring of any health and safety problems related to behavioral changes and treatment.
Baseline anthropometric measures were taken. The following BMI categories appropriate for Asians were used: underweight, BMI < 18.5 kg/m2 ; normal, 18.5 to 22.9 kg/m2; overweight, 23.0 to 24.9 kg/m2; obese class I, 25.0 to 29.9 kg/m2; obese class II BMI ≥ 30.0 kg/m2.17 BMI was measured every week to assess the immediate effect of lifestyle modification.
For 12 weeks, all subjects followed a weight maintenance diet (total calories per day divided into 55 to 60% carbohydrate, 15 to 20% protein and 20 to 25% fat) and moderate exercise in accordance with recommendations from the Endocrinology Association of Indonesia.18 All subjects were free living and consumed self-selected foods from a list of food replacements made according to their individual dietary habits. The dietitian reviewed the participants’ diet on a weekly basis to ensure compliance.
The exercise program consisted of moderate aerobic exercise at least 3 times per week, with a minimum of 30 minutes for each session.18 Each session included 5 minutes of warm-up, 20 minutes of main exercises, and 5 minutes of relaxation exercises. Each training session was supervised by a physiotherapist.
Blood pressure was averaged from two measurements using a mercurial sphygmomanometer after a 10 minute rest. All subjects reported for blood sampling in the morning after an overnight fast. Blood samples were centrifuged for 15 minutes, after which plasma- and serum-containing tubes were stored at -20°C until analysis. Blood glucose was measured by photometer autoanalyzer Modular P800. Plasma HDL-C, LDL-C and TG were measured using ARCHITECT ci8200 (Abbott Diagnostics, USA). High-sensitivity CRP was measured by sensitive immunoassay using Immulite® 1000 Analyzer System (Siemens Healthcare, Germany). HbA1c measurement was done by high-performance liquid chromatography (HPLC) using D-10™ (Bio-Rad, USA). Homeostatic model assessment of insulin resistance (HOMA-IR) was computed using the formula:
HOMA-IR = FPG x fasting serum insulin / 22.5
where FPG is expressed in mmol/L and fasting serum insulin in mU/L. Fetuin-A determination was performed by human fetuin-A enzyme immunoassay.
Data were presented as mean ± SD. Normality assumption of data from the placebo group and metformin group was evaluated and confirmed using Shapiro-Wilk normality test. Differences between and within groups were tested using the dependent t-test and independent sample t-test. Abnormal data were tested using Mann-Whitney U test, Wilcoxon test and Spearman’s correlation coefficient test. Two-sided p-values of less than 0.05 were regarded as statistically significant. The data were analyzed using SPSS software.
The local Ethics Committee approved the study.
Results
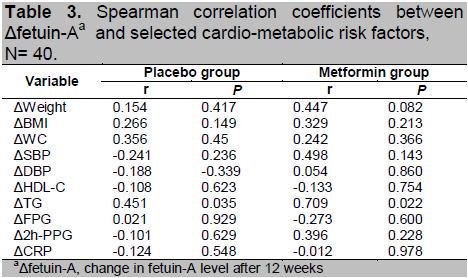
All 40 participants completed the study for 12 weeks. Analysis of baseline characteristics showed no significant differences in selected cardio-metabolic risk factors and fetuin-A (Table 1).
Click here to download Table 1
Table 1. Baseline characteristics of study subjects,
N= 40.
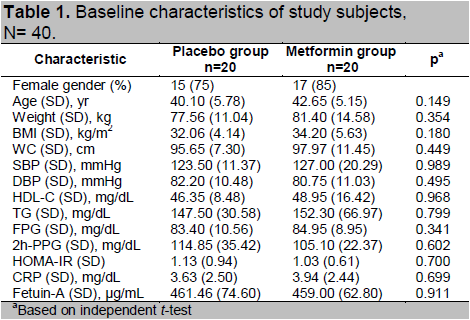
After 12 weeks, both groups had reductions in weight, BMI, WC, SBP, and DBP. Reduction in CRP was also found in the placebo group; fetuin-A was reduced in the metformin group. Compared to placebo, weight, BMI, WC, FPG, 2h-PPG, HDL-C and TG had decreased significantly in the metformin group (Table 2).
Click here to download Table2
Table 2. Change in selected cardio-metabolic risk factors from baseline and at 12 weeks, N= 40.
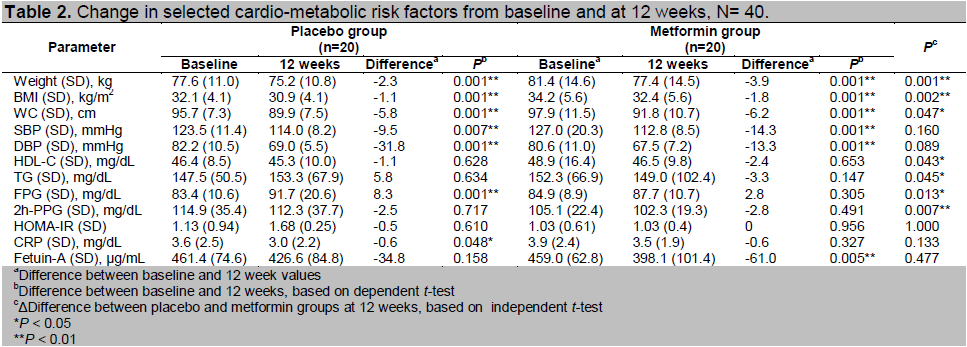
We analyzed the correlation between the change in serum fetuin-A levels after 12 weeks (Δfetuin-A) with changes in selected cardio-metabolic risk factors. Decrease in fetuin-A level was associated with a reduction in TG (ΔTG) in the metformin group (Table 3).
Click here to download Table 3
Table 3. Spearman correlation coefficients between Δfetuin-Aa and selected cardio-metabolic risk factors,
N= 40.
Obesity is the most common risk factor for the MetS and NAFLD.19 As suggested by novel evidence, hepatocytes from fatty liver release factors called hepatokines (e.g., fetuin-A, sex hormone-binding globulin) into the circulation that are directly involved in local pathogenesis, systemic inflammation and hepatic insulin resistance.2 The fetuin-A levels of obese children are apparently similar to those of adults. 20 A study by Mori and colleagues did not find a significant association between fetuin-A and insulin resistance in type 2 diabetic subjects.6 In contrast, other studies demonstrated a relationship between fetuin-A and insulin resistance in adults without T2D.4 Fetuin-A concentrations decreased significantly in obese children after substantial weight loss after 1 year, but was apparently unchanged in those who did not lose weight.20 Our study found that fetuin-A decreased significantly with lifestyle modification and metformin treatment for 12 weeks, possibly associated with weight reduction.
A recent systematic review showed a dose-response effect of aerobic exercise on visceral adiposity, but the ability of exercise to reduce visceral adipose tissue was less robust in those with metabolic disorders.21 It remains unclear if the same dose-response effect on central adiposity will also be seen in those with MetS. Nevertheless, during weight maintenance, regular exercise still has an important role in abdominal fat loss and may help prevent weight regain in those who have successfully lost weight.22 However, even in the absence of weight loss, exercise has been shown to reduce visceral adipose tissue.23 Our study demonstrated that weight, BMI and WC decreased significantly in the course of 12 weeks of lifestyle modification in both groups.
The National Cholesterol Education Program Adult Treatment Panel III (NCEP:ATP-III) recommends LDL-C reduction as the primary treatment goal for CVD risk reduction. Therapeutic lifestyle changes, particularly improvement in physical activity and weight management, need to be instituted in those individuals with the MetS to address elevated TG and low HDL-C.24 Although aerobic exercise training has generally been shown to increase HDL-C and decrease TG, its effects on LDL-C has been mixed.25,26 Beneficial effects of exercise training on lipids and lipoproteins may have additional impact when combined with dietary modification and weight loss.27 Our study demonstrated HDL-C and TG did not decrease significantly in the course of 12 weeks of lifestyle modification on both groups.
A recent meta-analysis of randomized controlled trials studying the effect of aerobic exercise on BP showed reduction in systolic and diastolic BP by approximately 3.8 and 2.6 mmHg, respectively.28 Although the effect of aerobic exercise on blood pressure is small and not consistently observed in all studies, there may be additional benefit when combined with dietary modification and/or weight loss.29 Our study demonstrated significant reductions in systolic and diastolic BP in both groups in the course of 12 weeks of lifestyle modification.
Insulin resistance is another core component of MetS that requires careful attention. Weight loss and lifestyle modification can lead to clinically meaningful improvements in insulin sensitivity and should be considered the primary therapeutic options for treating insulin resistance. The difficulties and frustrations associated with weight loss efforts and lifestyle modification have driven the demand for using pharmaceutical agents that target insulin resistance in a more direct fashion. The exact role for these agents is less clear. Several randomized controlled trials have shown that agents targeting insulin resistance can help prevent the progression to T2D in individuals with impaired glucose tolerance (IGT). These studies did not directly target individuals with the MetS. It is unclear whether these agents truly prevent progression to T2D or simply treat glucose intolerance or mild hyperglycemia. In addition, studies have not clearly shown whether these agents improve cardiovascular outcomes. As with weight loss medications, the goals for the use of agents targeting insulin resistance must be clear.30 Our study demonstrated HOMA-IR did not decrease significantly in both groups.
Fetuin-A induces low grade inflammation, which is also associated with MetS and an atherogenic lipid profile.19,31 Inflammation assessed by elevated CRP measurements has been linked to excess cardiovascular risk and MetS.32,33 CRP is a general marker of inflammation, making it suitable to assess in individuals with the metabolic syndrome. Elevated levels of CRP are associated with increased WC, insulin resistance, BMI and hyperglycemia; and in the presence of more components of the MetS.34-38 Because MetS has been linked with a greater chance of future cardiovascular events, CRP levels may be an important independent predictor of unfavorable outcomes in those already with MetS.39 There are, however, no currently recommended direct therapies targeting inflammation. Lifestyle modification and weight loss result in decreased CRP concentrations, as does the treatment of the other associated comorbidities such as dyslipidemia, elevated blood pressure, insulin resistance and hyperglycemia. 40,41 Our study observed CRP decreased significantly only in the placebo group.
Metformin has a primary mechanism of action of reducing hepatic glucose production. It has been shown to reduce the progression of diabetes in patients with IGT by approximately 31%, of which 53% had MetS.42 Incidence of MetS was also decreased by 17% in the metformin-treated group in the same study, which was driven primarily by improvements in WC and fasting glucose.43 Selected cardio-metabolic risk factors, however, did not improve with metformin to the same degree as with intensive lifestyle modification.44
Several clinical trials have supported the beneficial role of metformin in patients with NAFLD. Most of these studies have evaluated the effect of various doses of metformin on liver biochemistry (aminotransferase profile), histology, and MetS features.4 Some studies have suggested that metformin might be of benefit in the treatment of NAFLD, even in non-diabetic patients, when given with hypocaloric diet and weight control. However, the heterogeneity of these studies still prevents us from reaching firm conclusions about its expanded use. Moreover, metformin could have beneficial tissue-specific effects in NAFLD patients irrespective of its effects as insulin sensitizer.45 Our study showed that fetuin-A decreased significantly in the metformin group in the course of 12 weeks of treatment. The selected cardio-metabolic risk factors were significantly different between the placebo and metformin groups.
Lifestyle modification decreased human fetuin-A concentrations and selected cardio-metabolic risk factors in this 12-week study. These findings raise the possibility that fetuin-A may directly promote the MetS phenotype in humans. The selected cardio-metabolic factors significantly improved with metformin to the same degree as with the lifestyle modification. Longitudinal and larger scale studies are needed to evaluate the direction of the observed associations, the regulatory factors that alter serum fetuin-A concentrations, its effects on cardiovascular events, and the long-term effects of metformin on selected cardio-metabolic risk factors.
1. Bruce KD, Byrne CD. The metabolic syndrome: Common origins of a multifactorial disorder. Postgrad Med J. 2009;85(1009):614-21.
2. Reaven GM. Banting lecture 1988. Role of insulin resistance in human disease. Diabetes. 1988;37(12):1595-607.
3. Srinivas PR, Wagner AS, Reddy LV, et al. Serum alpha 2-HS-glycoprotein is an inhibitor of the human insulin receptor at the tyrosine kinase level. Mol Endocrinol. 1993;7(11):1445-55.
4. Stefan N, Hennige AM, Staiger H, et al. Alpha 2-Heremans-Schmid glycoprotein/fetuin-A is associated with insulin resistance and fat accumulation in the liver in humans. Diabetes Care. 2006;29(4):853-7.
5. Ix JH, Wassel CL, Chertow GM, et al. Fetuin-A and change in body composition in older persons. J Clin Endocrinol Metab. 2009;94(11):4492-8.
6. Mori K, Emoto M, Yokoyama H, et al. Association of serum fetuin-A with insulin resistance in type 2 diabetic and nondiabetic subjects. Diabetes Care. 2006;29(2):468.
7. Eckel RH, Grundy SM, Zimmet PZ. The metabolic syndrome. Lancet. 2005;365(9468):1415-28.
8. Miller WC, Koceja DM, Hamilton EJ. A meta-analysis of the past 25 years of weight loss research using diet, exercise or diet plus exercise intervention. Int J Obes Relat Metab Disord. 1997;21(10):941-7.
9. UK Prospective Diabetes Study (UKPDS) Group. Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). UK Prospective Diabetes Study (UKPDS) Group. Lancet. 1998;352(9131):854-65.
10. Sharoff CG, Hagobian TA, Malin SK, et al. Combining short-term metformin treatment and one bout of exercise does not increase insulin action in insulin-resistant individuals. Am J Physiol Endocrinol Metab. 2010;298(4):E815-23.
11. Glueck CJ, Fontaine RN, Wang P, et al. Metformin reduces weight, centripetal obesity, insulin, leptin, and low-density lipoprotein cholesterol in nondiabetic, morbidly obese subjects with body mass index greater than 30. Metabolism. 2001;50(7):856-61.
12. Wulffelé MG, Kooy A, de Zeeuw D, et al. The effect of metformin on blood pressure, plasma cholesterol and triglycerides in type 2 diabetes mellitus: A systematic review. J Intern Med. 2004;256(1):1-14.
13. Pasquali R, Gambineri A, Biscotti D, et al. Effect of long-term treatment with metformin added to hypocaloric diet on body composition, fat distribution, and androgen and insulin levels in abdominally obese women with and without the polycystic ovary syndrome. J Clin Endocrinol Metab. 2000;85(8):2767-74.
14. Lin HZ, Yang SQ, Chuckaree C, et al. Metformin reverses fatty liver disease in obese, leptin-deficient mice. Nat Med. 2000;6(9):998-1003.
15. WHO Expert Consultation. Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet. 2004;363(9403):157-63.
16. International Diabetes Federation. The IDF consensus worldwide definition of the metabolic syndrome. Brussels: International Diabetes Federation, 2006. http://www.idf.org/webdata/docs/ IDF_Meta_def_final.pdf.
17. Misra A, Misra R, Wijesuriya M. The metabolic syndrome in South Asians. In: Mohan V, Rao Gundu HR, eds. Type 2 diabetes in South Asians. Epidemiology, risk factors and prevention. New Delhi: Jaypee Bros., 2007;76-96.
18. Perkeni. Konsensus Pengelolaan dan Pencegahan Diabetes Mellitus Tipe 2 Di Indonesia, 2011. Perkumpulan Endokrinologi Indonesia.
19. Grundy SM. Obesity, metabolic syndrome, and cardiovascular disease. J Clin Endocrinol Metab. 2004;89(6):2595-600.
20. Reinehr T, Roth CL. Fetuin-A and its relation to metabolic syndrome and fatty liver disease in obese children before and after weight loss. J Clin Endocrinol Metab. 2008;93(11):4479-85.
21. Ohkawara K, Tanaka S, Miyachi M, et al. A dose-response relation between aerobic exercise and visceral fat reduction: Systematic review of clinical trials. Int J Obes. 2007;31(12):1786-97.
22. Wing RR, Hill JO. Successful weight loss maintenance. Ann Rev Nutr 2001;21:323-41.
23. Ross R, Janssen I, Dawson J, et al. Exercise-induced reduction in obesity and insulin resistance in women: a randomized controlled trial. Obes Res. 2004;12(5):789-98.
24. Stone NJ, Bilek S, Rosenbaum S. Recent National Cholesterol Education Program Adult Treatment Panel III update: adjustments and options. Am J Cardiol. 2005;96(4A):53E-9E.
25. Kelley GA, Kelley KS. Effects of aerobic exercise on lipids and lipoproteins in adults with type 2 diabetes: A meta-analysis of randomized-controlled trials. Public Health. 2007;121(9):643-55.
26. Kodama S, Tanaka S, Saito K, et al. Effect of aerobic exercise training on serum levels of high-density lipoprotein cholesterol: A meta-analysis. Arch Intern Med. 2007;167(10): 999-1008.
27. Stefanick ML, Mackey S, Sheehan M, et al. Effects of diet and exercise in men and postmenopausal women with low levels of HDL-C cholesterol and high levels of LDL-C cholesterol. N Engl J Med. 1998;339(1):12-20.
28. Whelton SP, Chin A, Xin X, et al. Effect of aerobic exercise on blood pressure: A meta-analysis of randomized, controlled trials. Ann Intern Med. 2002;136(7):493-503.
29. Bacon SL, Sherwood A, Hinderliter A, et al. Effects of exercise, diet and weight loss on high blood pressure. Sports Med. 2004;34(5):307-16.
30. Cornier MA, Dabelea D, Hernandez TL et al. The metabolic syndrome. Endocrine Rev. 2008;29(7):777-822.
31. Hennige AM, Staiger H, Wicke C, et al. Fetuin-A induces cytokine expression and suppresses adiponectin production. PLoS One. 2008;3(3):e1765.
32. Ridker PM. High-sensitivity C-reactive protein: Potential adjunct for global risk assessment in the primary prevention of cardiovascular disease. Circulation. 2001;103(13):1813-8.
33. Ridker PM, Buring JE, Cook NR, et al. C-reactive protein, the metabolic syndrome, and risk of incident cardiovascular events: an 8-year follow-up of 14719 initially healthy American women. Circulation. 2003;107(3):391-7.
34. González AS, Guerrero DB, Soto MB, et al. Metabolic syndrome, insulin resistance and the inflammation markers C-reactive protein and ferritin. Eur J Clin Nutr. 2006;60(6):802-9.
35. Deepa R, Velmurugan K, Arvind Ket, al. Serum levels of interleukin 6, C-reactive protein, vascular cell adhesion molecule 1, and monocyte chemotactic protein 1 in relation to insulin resistance and glucose intolerance—The Chennai Urban Rural Epidemiology Study (CURES). Metabolism. 2006;55(9):1232-8.
36. Guldiken S, Demir M, Arikan E, et al. The levels of circulating markers of atherosclerosis and inflammation in subjects with different degrees of body mass index: Soluble CD40 ligand and high-sensitivity C-reactive protein. Thromb Res. 2007;119(1):79-84.
37. Bahia L, Aguiar LG, Villela N, et al. Relationship between adipokines, inflammation, and vascular reactivity in lean controls and obese subjects with metabolic syndrome. Clinics (Sao Paulo). 2006;61(5):433-40.
38. González AS, Guerrero DB, Soto MB, et al. Metabolic syndrome, insulin resistance and the inflammation markers C-reactive protein and ferritin. Eur J Clin Nutr. 2006;60(6):802-9.
39. Clearfield MB. C-reactive protein: A new risk assessment tool for cardiovascular disease. J Am Osteopath Assoc. 2005;105(9):409-16.
40. van Dielen FM, Buurman WA, Hadfoune M, et al. Macrophage inhibitory factor, plasminogen activator inhibitor-1, other acute phase proteins, and inflammatory mediators normalize as a result of weight loss in morbidly obese subjects treated with gastric restrictive surgery. J Clin Endocrinol Metab. 2004;89:4062-8.
41. Devaraj S, Rogers J, Jialal I. Statins and biomarkers of inflammation. Curr Atheroscler Rep. 2007;9(1):33-41.
42. Knowler WC, Barrett-Connor E, Fowler SE, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346(6):393-403.
43. Orchard TJ, Temprosa M, Goldberg R, et al. The effect of metformin and intensive lifestyle intervention on the metabolic syndrome: The Diabetes Prevention Program randomized trial. Ann Intern Med. 2005;142(8):611-9.
44. Ratner R, Goldberg R, Haffner S, et al. Impact of intensive lifestyle and metformin therapy on cardiovascular disease risk factors in the diabetes prevention program. Diabetes Care. 2005;28(4):888-94.
45. Mazza A, Fruci B, Garinis GA, et al. The role of metformin in the management of NAFLD. Exp Diabetes Res. 2012;2012:716404.
Authors are required to accomplish, sign and submit scanned copies of the JAFES Declaration that the article represents original material that is not being considered for publication or has not been published or accepted for publication elsewhere.
Consent forms, as appropriate, have been secured for the publication of information about patients; otherwise, authors declared that all means have been exhausted for securing such consent.
The authors have signed disclosures that there are no financial or other relationships that might lead to a conflict of interest. All authors are required to submit Authorship Certifications that the manuscript has been read and approved by all authors, and that the requirements for authorship have been met by each author.