The Philippine Clinical Practice Guidelines (CPG) for the management of dyslipidemia was published in July 2005. The guidelines, based on the best available evidence at that time, was part of the strategy to improve cardiovascular disease (CVD) risk and dyslipidemia management that is more appropriate and relevant for Filipinos. It took into consideration the Philippines? demographic, socioeconomic and health situation. The CPG also dealt with equity issues and considered the disadvantaged groups who live below the annual poverty threshold, those who cannot afford laboratory examinations and drug therapy and have limited or no access to health care or are undernourished. The guidelines were not meant to be viewed as absolute rules and the recommendations should supplement, but not replace, sound clinical judgment.
The Philippine CPGs' stated objectives were to develop valid and applicable dyslipidemia CPGs for Filipinos with special consideration for existing health inequities. The CPG considered lifestyle and diet modification as the cornerstone for total CVD risk management as well as primary and secondary prevention of dyslipidemia. The guidelines also outlined screening and drug therapy recommendations, as well as considerations for the disadvantaged population.
The guidelines were spearheaded by the Philippine Heart Association (PHA) Council on Preventive Cardiology in cooperation with partner organizations. Technical and financial support was provided by the International Clinical Epidemiology Network (INCLEN). A technical research committee comprising cardiologists, endocrinologists, social scientists and epidemiologists was formed. Panelists were composed of appointed representatives of partner organizations and government stakeholders.
The Philippine CPG on Dyslipidemia were developed using the INCLEN Guideline Development Cycle, referred to as the Knowledge Management Plus (KM+).1 In this development cycle, clinical trial evidence available at that time were appraised for validity as well as applicability to the Philippine setting. The quality of evidence was graded as high, moderate, low or very low. Evaluation of treatment benefit and harm were prepared from data in literature following the KM+ process and were determined as net benefits (the intervention clearly does more good than harm), trade-offs (trade-offs exist between benefit and harm), uncertain net benefits (uncertainties whether the intervention does more good than harm) or no net benefits (the intervention clearly does not do more good than harm). After the evaluation of intervention was described, crude cost benefit was analyzed and a balance of net benefits and costs were made to determine if incremental health benefits were worth the costs. Recommendations formulated following the KM+ process included: Do it, Probably do it, No recommendation, Probably don't do it or Don't do it.
Click here to download Table 1Table 1. INCLEN Guideline Development Cycle Knowledge Management Plus (KM+)1

This paper summarizes the guidelines of 2005 and the basis and quality of evidence for each recommendation.
Cardiovascular disease is the leading cause of mortality in the Philippines.2 In 2009, the cause-specific mortality rate for diseases of the heart was 109.4 deaths/100,000 population and 71.0 deaths/100,000 population for diseases of the vascular system.1 This was much higher compared to the report in the CPG of 78.4 and 58.4 deaths/100,000 population in 1999.
The reported prevalence for atherosclerosis-related risk factors is also increasing. In the NNHES survey in 2008, the prevalence of dyslipidemia was 72% which was higher than the survey done in 2003 (62%).3,4 In both surveys, the prevalence of dyslipidemia was caused by a high prevalence of low HDL-C levels.
The CPG in 2005 recommended that all patients regardless of their risk profile should be advised on lifestyle modification including smoking cessation, maintenance of target weight and prevention of obesity, regular physical activity and adequate blood pressure monitoring and control to reduce their overall risk.
Lifestyle Management Recommendations- Smoking.
There is an increased cardiovascular risk associated with smoking in a dose dependent manner.5 To be able to reduce this risk, the CPG recommended complete smoking cessation and passive smoking avoidance.
- Weight Management.
Abdominal Obesity is more strongly correlated with CV risk than BMI.6-8 The CPG recommended the use of waist circumference as the measure of abdominal obesity rather than waist-hip circumference ratio (WHR). This is to be used as a tool to assess a patient's risk associated with co-morbidities such as diabetes, metabolic syndrome, CHD, sleep apnea and osteoarthritis.8
- Physical Activity
Regular physical activity is associated with lower levels of LDL and triglycerides, higher HDL cholesterol, improved insulin sensitivity and lower blood pressure.9-12 It is also associated with lower risk of CVD and CHD.13-18 The CPG therefore recommended that for physical activity to be protective, it should be moderately vigorous, done 3x a week or an activity equivalent to 3,500 kilocalories.18 Examples of these types of activities included swimming, basketball, volleyball, badminton, tennis, jogging and running. Walking for 56 km (35 miles) or climbing 438 flights of stairs with 20 steps per flight is equivalent to 3,500 kilocalories.
Hypertension is an associated condition in patients with hypercholesterolemia.19-20 The CPG recommended that regular blood pressure screening should be done in apparently healthy individuals.
At the time of publication, evidence for dietary interventions showed cardioprotection.21-23 Advice on dietary interventions was considered cost effective and highly recommended. The CPG enumerated that:
Low fat,low cholesterol diet is recommended for life for patients at any level of cardiovascular risk especially in patients with established atherosclerosis.
Low fat, low cholesterol diet can still achieve correction of nutritional deficiencies among poorly nourished and elderly patients.
Use of the Food and Nutrition Research Institute of the Department of Science and Technology (FNRI-DOST) Simple Dietary Plan for Fat Modification (2000)24 pointers in planning meals:
Choose freely from fruits, vegetables, cereals, root crops, bread, dried beans and nuts.
Use fish as main dish at least 3 times a week.
May eat chicken meat as a substitute to fish at least three to four times a week. Eat chicken preferably without skin.
For other kinds of meat, use lean parts and prepare as boiled, baked, broiled or roasted. Trim off any visible fat.
Use evaporated filled milk or skimmed milk instead of whole milk and avoid whole milk products such as cheese, butter, cream. Use margarine in moderation instead of butter.
Use polyunsaturated fats and oils such as olive oil, corn oil, canola oil and peanut oil.
Limit egg yolk consumption to only three pieces per week.
Avoid rich desserts such as cakes, pastries, cookies, pies, ice cream and chocolates.
Always read the nutrition labels of packaged/processed foods.
The CPG stated that for low risk patients without evidence of atherosclerosis, drug therapy is not recommended regardless of lipid levels.
The 2 statin trials (AFCAPS/TexCAPS, WOSCOPS), 1 fibrate trial (HHS) and 1 cholestyramine trial (LRC-CPPT) that were included in the appraisal showed problems of applicability to Filipinos which lowered the quality of evidence.25-28 Crude Cost benefit analysis showed that to prevent 1 CV death, 1-3 MIs, 2-3 CV events and one revascularization, it would cost PhP 12.3 M to treat 286 Filipinos with a statin. Moreover, treating the same number of patients with a fibrate would cost PhP 12.7 M to prevent 2 MIs.
On this basis, the CPG recommended against drug therapy for primary prevention because of the poor benefit and high cost of treatment. However, for patients with familial hypercholesterolemia, treatment is recommended even without risk factors.
The CPG stated that statins MAY BE recommended among patients without established atherosclerosis but with more than 3 risk factors and total cholesterol >190 mg/dl or LDL >100 mg/dl.
Established atherosclerosis referred to patients with acute coronary syndrome, previous MI or unstable angina, peripheral arterial disease, stroke or transient ischemic attack and evidence of coronary artery disease or revascularization.
The evidence for this recommendation was based on 2 statin trials (ASCOT-LLA and ALLHAT-LLT).29-30 ASCOT-LLA showed that statin therapy significantly reduced MI (37%), stroke (27%) and CV events (28%) among hypertensive patients with more than 3 additional risk factors and no established CVD. ALL-HAT LLT on the other hand did not demonstrate important clinical outcomes. Even if the ASCOT-LLA demonstrated benefits, the CPG rated the evidence quality as low because of epidemiological and socioeconomic issues that are not applicable to the Filipinos thus benefit was judged as "uncertain."
With little evidence to work with, the basis for this recommendation was expert opinion, statins remain an option and was given the recommendation "probably do it."
- Diabetic Patients
The CPG recommended statins for diabetic patients without evidence of atherosclerosis and with total cholesterol >190 mg/dl or LDL <100 mg/dl.
The basis for the above recommendation came from 4 statin trials (AFCAPS/TexCAPS, ASCOT-LLA, HPS, PROSPER) with large diabetic cohorts and 1 trial (CARDS) designed for diabetic patients with dyslipidemia.25,29,31-33 The pooled results of these trials showed reduction in fatal and nonfatal MI (34%), revascularization (15%) and CV events (28) though no significant reductions in mortality. The CPG rated the quality of evidence as moderate and treatment thresholds were based on baseline levels in HPS.
Cost analysis showed that to prevent 1 MI, revascularization and CV event, it would cost PhP 3.9-5.3 M to treat 90 diabetic patients, thus the CPG recommendation for statins was "do it."
The CPG stated that for diabetic patients fibrates MAY BE recommended as an alternative to statins in those with HDL <35 mg/dl and LDL < 90mg/dl.
The CPG's recommendation for fibrates as "Probably do it" was considered beneficial to patients with low HDL given its mechanism of action. Clinical trials appraised and evaluated for this recommendation included the Diabetes Atherosclerosis Intervention Study (DAIS) and the St. Mary's, Ealing, Northwick, Park Diabetes Cardiovascular Disease Prevention (SEND CAP) Study.34-35
The CPG recommended that for patients with established atherosclerosis and total cholesterol > 190 mg/dL or LDL >100 mg/dL, statins are indicated.
Evidence included in the appraisal for the use of statin in secondary prevention was the Heart Protection Study (HPS)36 where crude cost analysis showed that the five year therapy with statins will amount to PhP 2.9 million to prevent one death, four CV events, one MI, one stroke and one revascularization.
The CPG stated that fibrates may be recommended as an alternative to statins if HDL levels are <35 mg/dL and LDL <90 mg/dL.
The evidence for fibrates were from the Veteran's Affairs High-Density Lipoprotein Intervention Trial (VA-HIT)37 and the Bezafibrate Infarction Prevention (BIP)38 Trial where crude cost-analysis indicated that fibrates taken five to six years would cost approximately PhP 2.1 to 2.7 million to prevent one MI and one stroke.
Similar to the statin group, the quality of evidence was downgraded due to the difference in socioeconomic characteristics between populations. After considering costs and the marginal benefit of fibrate therapy, the consensus recommendation was to limit fibrates as an alternative to statins.
The CPG elucidated that in patients without risk factors (Table 2), history or symptoms of established atherosclerosis, screening of lipid levels was not recommended.
The only recommended intervention for such patients were non-pharmacological which should be instituted regardless of lipid levels.
Clinical evidence indicated benefit of statin therapy in patients with multiple risk factors, specifically in patients with total cholesterol >190 mg/dL or LDL >100 mg/dL. Therefore, the panel recommended determining lipid levels for screening purposes in patients with multiple risk factors but no established atherosclerosis. Total cholesterol was also considered a relevant screening parameter based on the Anglo-Scandinavian Cardiac Outcome Trial-Lipid Lowering Arm (ASCOT-LLA)39 trial.
The CPG advocated that in patients with established atherosclerosis or diabetes, screening with lipid profile is recommended.
-
The panel recommended statin therapy for patients with atherosclerosis or diabetes if total cholesterol > 190 mg/dL or LDL > 100 mg/dL and fibrate therapy if HDL <35 mg/dL and LDL < 90 mg/dL. Lipid profiling was deemed appropriate in identifying patients where pharmacotherapy was indicated.
Table 2. Risk Factors1
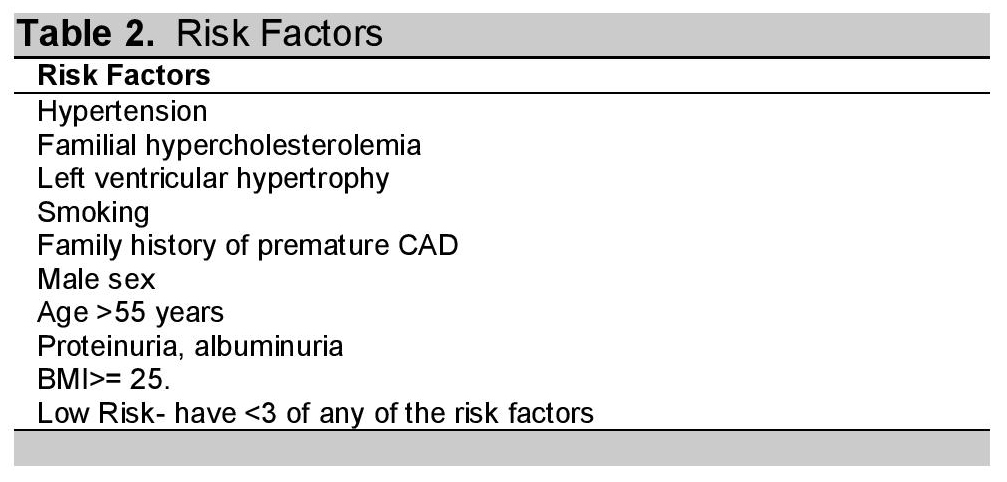
The CPG gave the following recommendations in the initiation of pharmacotherapy:
Statin therapy may be initiated in patients with no established atherosclerosis but have > 3 risk factors and total cholesterol >190 mg/dL or LDL >100 mg/dL.
Statin therapy initiation is recommended for diabetics with no established atherosclerosis but with total cholesterol >190 mg/dL or LDL >100 mg/dL.
Statin therapy initiation is recommended for patients with established atherosclerosis and with total cholesterol >190 mg/dL or LDL >100 mg/dL. However, costs should be considered for the underprivileged. For patients who opt to defer screening, the initiation of statin therapy may still be given as an option after proper patient education (informed patient choice).
Fibrates may be initiated as an alternative to statins in diabetics patients with no established atherosclerosis if HDL <35 mg/dL and LDL <90 mg/dL.
The CPG gave the following targets for LDL-C:
A 30 to 40% LDL reduction from baseline OR
< 77 mg/dL
The CPG endorsed a 30% to 40% LDL reduction from baseline as a suitable treatment goal based on significant risk reductions in randomized clinical trials25,33,39-44 with approximately 30 to 40% LDL reduction from baseline.
The CPG recommended <77 mg/dL as a target for treatment on the basis of the Treating to New Targets (TNT)45 trial where additional CV benefits were achieved when LDL was decreased to <77 mg/dL. Although TNT and PROVE-IT46 were appraised as having low evidence quality due to poor applicability to Filipinos, a significant benefit was observed making <77 mg/dL a suitable treatment goal in pharmacotherapy. Crude cost analysis showed that shifting patients from usual statin therapy to intensive therapy using atorvastatin 80 mg/day on 167 individuals for 3.45 years to reduce one stroke, two MIs and seven CV events require PhP 16.8 million.
Patient Monitoring
The CPG recommended the earliest time to repeat measurements of lipid profile is within six weeks after initiation of therapy. In patients where total cholesterol was used as a basis for initiating statin therapy, fixed dose was recommended and dose titration aim of at least a 20% reduction of total cholesterol from baseline.
The CPG considered disadvantaged patients as those who:
- Live below the annual poverty threshold of PhP 12,267.00 (as of 2003)
- Cannot afford laboratory examinations and drug therapy
- Have limited or no access to healthcare
- Are undernourished (i.e., People with BMI <18.5)
Recommendations for the disadvantaged population are similar to the general population with greater cost consideration for screening, medical therapy and lipid monitoring. Patients should be provided with proper and adequate information and education regarding options to be able to make an informed choice.
The strength of the Clinical Practice Guidelines for the management of dyslipidemia in the Philippines (2005) lies in the process by which recommendations were made, taking into full consideration the applicability of international clinical trials in the local setting and the cost-analysis of implementing these practices among Filipinos.
The recommendations of the 2005 Guidelines are currently being updated based on new clinical trial evidence using the same process of critical appraisal as the 2005 CPG. These updates will take into consideration existing international guidelines prioritizing its applicability in the Philippine setting. The updated version of the guidelines is expected to be available by 2015.
1. The International Clinical Epidemiology Network (INCLEN). Knowledge management plus: The INCLEN guideline development cycle. Available at www.inclentrust.org. Accessed on April 2014.
2. Health Statistics: Leading cause of mortality and morbidity. Department of Health Website. Available at http://www.doh.gov.ph/node/198.html. Accessed on April 15, 2014.
3. Sy RG, Morales DD, Dans AL, Paz-Pacheco E, Punzalan FER, Abelardo NS, Duante CA. Prevalence of atherosclerosis-related risk factors and diseases in the Philippines. J Epidemiol 2012;22(5):440-447. http://dx.doi.org/10.2188/jea.JE20110095.
4. Dans AL, Morales DD, Velandria F, Abola TB, Rozas JR, Punzalan FER, SYS Rg, Paz-Pacheco E, for the NNHes: 2003 Group. National Nutrition and Health Survey (NNHes): Atherosclerosis-related diseases and risk factors. Phil J Int Med 2005;43:103-115
5. Wilhemsen L. Coronary Heart Disease: Epidemiology of smoking and intervention studies of smoking. Am Heart J 1988;115:242-249.http://dx.doi.org/10.1016/0002-8703(88)90644-8.
6. The Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on detection Evaluation and Treatment of High Blood cholesterol in Adults (Adult Treatment Panel III). Bethesda, MD: National Institutes of Health; 2002. NIH Publication NO 02-5215.
7. Yusuf S, Hawken S, Ounpuu S, et al. Effect of modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): Case control study. Lancet. 2004-2005;364:937-952.
8. Regional Office for the Western Pacific of the World Health Organization, the International Association for the Study of Obesity and the International Obesity Task Force. The Asia-Pacific perspective: Redefining obesity and its treatment. Health Communications Australia Pty Limited. February 2000.
9. Blair SN, Cooper KH, Gibbons LW, et al. Changes in coronary heart disease risk factors associated with increased treadmill time in 753 men. N Eng J Med. 1993;328:538-545.
10.King H, Krista AM. Prevention of Type II diabetes in physical training: Epidemiological considerations and study methods. Diabetes Care. 1992;15 (Supp 4): 1794-1799.
p style="text-align:justify"> 11. Helmrich Sp, Ragland DR, Leung RW, Paffenbarger RS Jr. Physical activity and reduced occurrence of non-insulin dependent diabetes mellitus. N Eng J Med. 1991; 325:147-152.12. Haskell WL, Akderman EL, Fair JM et al. Effects of multiple risk factor reduction in coronary atherosclerosis and clinical cardiac events in men and women with coronary artery disease: The Stanford Coronary Risk Intervention Project (SCRIP). Circulation 1994;89:975-990. http://dx.doi.org/10.1161/01.CIR.89.3.975
13. Leon AS, Connett J, Jacobs DR Jr., et al. Leisure time physical activity levels and risk of coronary heart disease and death: The Multiple Risk Factor Intervention Trial. JAMA. 1987;258:2388-2395. http://dx.doi.org/10.1001/jama.1987.03400170074026.
14. Ekelund LG, Haskell WL, Johnson JL, et al. Physical Fitness as a predictor of cardiovascular mortality in asymptomatic North American men: The Lipid Research Clinics Mortality Follow-up study. N Engl J Med. 1988;319:1379-1384. http://dx.doi.org/10.1056/NEJM198811243192104.
15. Blain SN, Kohl HW 3rd, Paffenbarger RS Jr, et al. Physical Fitness and All Cause Mortality : A prospective study of healthy men and women. JAMA. 1989:262:2395-2401.
16. Morris JN, Clayton DG, Everitt MG, et al. Exercise in leisure time: Coronary attack and death rates. Br Heart J. 1990;63:325-332. http://dx.doi.org/10.1136/hrt.63.6.325.
17. Sandvik l, Erikssen J, Thaulow E, et al. Physical Fitness as a predictor of mortality among healthy, middle aged Norweigian men. N Engl J Med. 1993:328:533-537.
18. Paffenbarger RS Jr, Hyde RT, Wing AL, et al. The association of changes in physical activity level and other lifestyle characteristics with mortality in men. N Engl J Med. 1993;328:538-545. http://dx.doi.org/10.1056/NEJM199302253280804.
19. Working Group Report on Management of Patients with Hypertension and High Blood Cholesterol. National Working Group Programs Working Group Report on Management of Patients with Hypertension and High Blood Cholesterol. Ann Intern Med. 1991;114:224-237.
20. Cutler JA, Psaty BM, MacMahon S, et al. Public Health Issues in hypertension control: What has been learned from clinical trials. In : Laragh JH, Brenner BM, eds. Hypertension: pathophysiology, diagnosis and management. 2nd ed New York. Raven Press, 1995:253-270.
p style="text-align:justify"> 21. Hooper L, Summerbell CD, Higgins JPT, et al. Reduced or modified dietary fat for preventing cardiovascular disease (Cochrane Review). In: The Cochrane Library, Issue 2, 2004-2005. Chichester,UK; John Wiley & Sons Ltd.22. Clarke R, Frost C, Collins R, et al. Dietary lipids and blood cholesterol quantitative analysis of metabolic ward studies. BMJ. 1997;314:112-117. http://dx.doi.org/10.1136/bmj.314.7074.112.
23. Mersink RP, Katan MB. Effect of dietary fatty acids on serum lipid and lipoproteins. Arteriosclerosis Thrombosis. 1992;12:911-919. http://dx.doi.org/10.1161/01.ATV.12.8.911.
24. The Biomedical Nutrition Research Division, Food and Nutrition Research Institute Department of Science and Technology. Nutritional Guidelines for Filipinos: Revised Edition 2000. Taguig: Food and Nutrition Research Institute ; 2000.
25. Downs JR, Clearfield M, Weis S, et al. Primary prevention of acute coronary events with lovastatin in men and women with average cholesterol levels: Results of AFCAPS/TexCAPS. JAMA. 1998;279:1615-1622. http://dx.doi.org/10.1001/jama.279.20.1615.
26. Shepherd J, Cobbe SM, Ford I, et al. Prevention of coronary heart disease with pravastatin in men with hypercholesterolemia. N Engl J Med. 1995;333:1301-1307. http://dx.doi.org/10.1056/NEJM199511163332001.
27.Frick MH, Elo MO, Haapa K, et al. Helsinki Heart Study; primary prevention trial with gemfibrozil in middle aged men with dyslipidemia: Safety of treatment, changes in risk factors and incidence of coronary heart disease. N Engl J Med. 1987;317:1237-1245. http://dx.doi.org/10.1056/NEJM198711123172001.
28. The Lipid Research Clinics Coronary Primary Prevention Trial Results II. The relationship of the reduction in incidence of coronary heart disease. JAMA.1984;251:351-364
29. ever PS, Dahlof B, Poulter NR, et al. Prevention of coronary and stroke events with atorvastatin in hypertensive patients who have average or lower than average cholesterol concentrations, in the Anglo-Scandinavian Cardiac Outcome Trial-Lipid Lowering Arm (ASCOT-LLA): A multicenter randomized controlled trial. Lancet. 2003;361:1149-1158. http://dx.doi.org/10.1016/S0140-6736(03)12948-0.
30. The ALLHAT Officers and Coordinators for the ALLHAT Collaborative Research Group. Major outcomes in moderately hypercholesterolemic, hypertensive patients randomized to pravastatin vs. usual care . The Anti-Hypertensive and Lipid Lowering Treatment to Prevent Heart Attack Trial. JAMA 2002;288:2998-3007.
p style="text-align:justify"> 31. Collins R, Armitage J, Parish S, et al. MRC/BHF Heart Protection Study of cholesterol-lowering with simvastatin in 5963 people with diabetes; A randomized placebo control trial. Lancet. 2003; 361;2005-2016.32. Shepherd J, Blauw GJ, Murphy MB et al. Pravastatin in elderly individuals at risk of vascular disease (PROSPER): A randomized control trial. Lancet. 2002; 360: 1623-1630. http://dx.doi.org/10.1016/S0140-6736(02)11600-X.
33. Calhoun HM, Betteridge DJ, Durrington PN, et al. Primary Prevention of cardiovascular disease with atorvastatin in type 2 diabetes in the Collaborative Atorvastatin Diabetes Study (CARDS); multicenter randomized placebo trial. Lancet. 2004-2005;364: 685-696.
34. Effect of fenofibrateon progression of coronary artery disease in type 2 diabetes: The Diabetes Atherosclerosis Intervention Study, a randomized study. Lancet. 2001;357:905-910. http://dx.doi.org/10.1016/S0140-6736(00)04209-4.
35. Elkeles RS, Diamond JR, Poulter C, et al. A double blind placebo controlled study of bezafibrate: the St Mary's, Ealing, Northwick, Park Diabetes Cardiovascular Disease Prevention (SEND CAP) Study . Diabetes Care. 1998;21: 641-648.http://dx.doi.org/10.2337/diacare.21.4.641.
36. Heart Protection Study Collaborative Group. MRC/BHF Heart Protection Study of cholesterol-lowering simvastatin in 5963 people with diabetes: A randomized placebo-controlled trial. Lancet. 2003; 361; 2005-2016. 37. Rubins HB, Robins SJ, Collins D, et al. Gemfibrozil for the secondary prevention of coronary heart disease in men with low levels of high-density lipoprotein cholesterol. Veterans Affairs High-Density Lipoprotein Cholesterol Intervention Trial Study Group. N Engl J Med. 1999; 341: 410-418. http://dx.doi.org/10.1056/NEJM199908053410604.
37. Rubins HB, Robins SJ, Collins D, et al. Gemfibrozil for the secondary prevention of coronary heart disease in men with low levels of high-density lipoprotein cholesterol. Veterans Affairs High-Density Lipoprotein Cholesterol Intervention Trial Study Group. N Engl J Med. 1999; 341: 410-418. http://dx.doi.org/10.1056/NEJM199908053410604.
38. Secondary prevention by raising HDL and reducing triglycerides in patients with coronary artery disease: The Bezafibrate Infarction Prevention (BIP) study. Circulation 2000; 101: 21-27. 39. Sever PS, Dahlof B, Poulter NR, et al. Prevention of coronary and stroke events with atorvastatin in hypertensive patients who have average or lower-than-average cholesterol concentrations, in the Anglo-Scandinavian Cardiac Outcome Trial-Lipid Lowering Arm (ASCOT-LLA): A multicenter randomized controlled trial. Lancet. 2003; 361: 1149-1158.
39.Sever PS, Dahlof B, Poulter NR, et al. Prevention of coronary and stroke events with atorvastatin in hypertensive patients who have average or lower-than-average cholesterol concentrations, in the Anglo-Scandinavian Cardiac Outcome Trial-Lipid Lowering Arm (ASCOT-LLA): A multicenter randomized controlled trial. Lancet. 2003; 361: 1149-1158. http://dx.doi.org/10.1016/S0140-6736(03)12948-0.
40. Snow V, Aronsosn MD, Hornbake ER, et al. Lipid control in the management of type 2 diabetes mellitus: A clinical practice guideline for the American College of Physicians. Ann Intern Med. 2004; 140: 644-649. http://dx.doi.org/10.7326/0003-4819-140-8-200404200-00012.
p style="text-align:justify"> 41. Heart Protection Study Collaborative Group. MRC/BHF Heart Protection Study of cholesterol-lowering simvastatin in 20536 high risk individuals: A randomized placebo-controlled trial. Lancet. 2002; 360; 7-22.42. Scandinavian Simvastatin Survival Study Group. Randomised trial of cholesterol lowering in 4,444 patients with coronary heart disease: The Scandinavian Simvastatin Survival Study (4S). Lancet. 1994; 344:1383-1389.
43.Long-Term Intervention with Pravastatin in Ischemic Disease (LIPID) Study Group. Prevention of Cardiovascular Events and death in pravastatin patients with coronary heart disease and a broad range of initial cholesterol levels. N Engl J Med. 1998; 339; 1349-1357.
44. Sacks FM, Pfeffer MA, Moye LA, et al. The effect of pravastatin on coronary events after myocardial infarction in patients with average cholesterol levels. N Eng J Med. 1996;335: 1001-1009. http://dx.doi.org/10.1056/NEJM199610033351401.
45. LaRosa JC, Grundy SM, Waters DD, et al. Intensive lipid lowering with atorvastatin in patients with stable coronary disease. N Engl J Med. 2005; 352:1425-1435. http://dx.doi.org/10.1056/NEJMoa050461.
46. Cannon CP, Braunwald E, McCabe CH, et al. Intensive versus moderate lipid lowering with statins after acute coronary syndromes. N Engl J Med. 2004; 350: 1495-1504. http://dx.doi.org/10.1056/NEJMoa040583.
Authors are required to accomplish, sign and submit scanned copies of the JAFES Declaration that the article represents original material that is not being considered for publication or has not been published or accepted for publication elsewhere.
Consent forms, as appropriate, have been secured for the publication of information about patients; otherwise, authors declared that all means have been exhausted for securing such consent.
The authors have signed disclosures that there are no financial or other relationships that might lead to a conflict of interest. All authors are required to submit Authorship Certifications that the manuscript has been read and approved by all authors, and that the requirements for authorship have been met by each author.