While sinus tachycardia, atrial fibrillation, and congestive heart failure are well-recognized cardiovascular manifestations of Graves' disease,1 pericardial effusion due to thyrotoxicosis is rarely reported. This case highlights the clinician's role in determining the association between the two disease entities.
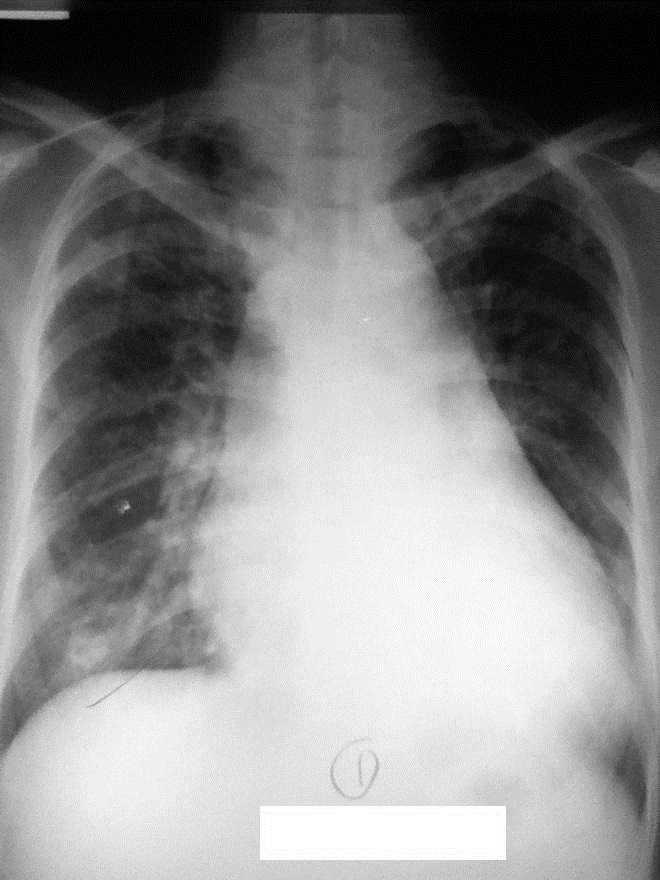
A 32-year-old Filipino woman on the 19th week of her fourth pregnancy presented at the emergency room with a seven-year history of diffuse goiter associated with tremors, palpitations, heat intolerance, exophthalmos and exertional dyspnea. Prior to this consult, she was previously taking anti-thyroid medications which were discontinued five years ago upon improvement of symptoms, and was subsequently lost to follow up. On admission, the patient was febrile, tachycardic in atrial fibrillation, hyperreflexic, with fine finger tremors. Ophthalmologic examination revealed positive Dalrymple's, Kocher's, and Von Graefe's signs, lagophthalmos, chemosis and periorbital edema. The thyroid gland was non-tender and diffusely enlarged, measuring 5x5 cm per lobe. Auscultation revealed bibasal fine crackles, and there was note of hyperpigmented, non-pitting induration over the pretibial area of both legs, clubbing of the fingers and pedal edema. She was treated for Graves' disease in thyroid storm, with congestive heart failure from thyrotoxic heart disease. Her chest radiograph showed left ventricular cardiomegaly (Figure 1). She improved with propylthiouracil, propranolol and furosemide and was discharged.
Click here to download Figure 1Figure 1.Chest radiograph taken on postero-anterior view during initial admission showing left ventricular cardiomegaly with pulmonary congestive changes.
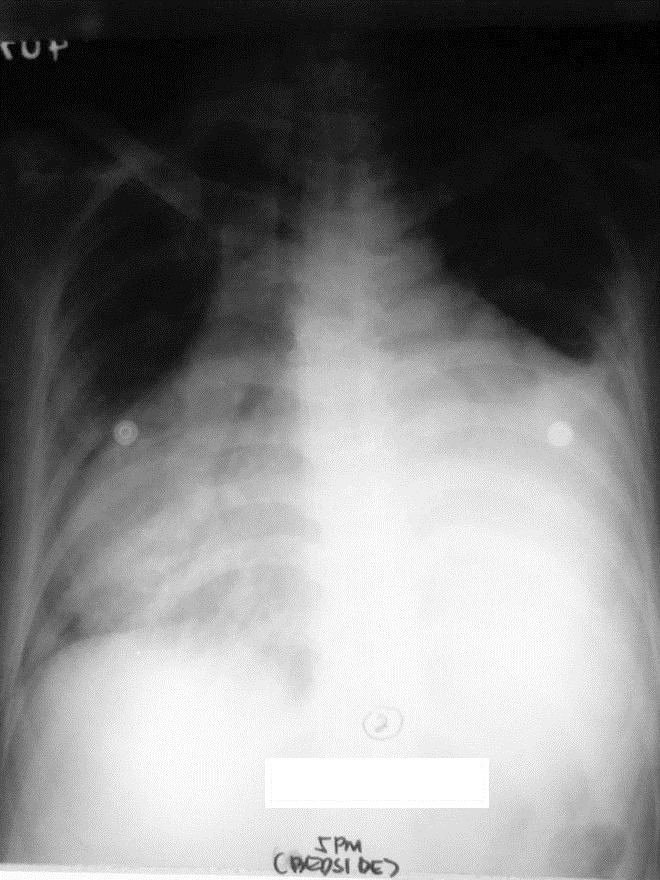
Nineteen days later, the patient noted recurrence of exertional dyspnea, bipedal edema and three-pillow orthopnea. No chest pain or fever was observed. Upon readmission, she was tachycardic with distended neck veins, muffled heart sounds and Grade 2 bipedal edema. Deep tendon reflexes were normal with no tremors. Chest radiograph now revealed multichambered cardiomegaly (Figure 2.)
Click here to download Figure 2Figure 2. Chest radiograph on readmission showing multi-chambered cardiomegaly and characteristic water-bottle sign indicative of massive pericardial effusion.
During the patient's first admission, free thyroxine (fT4) levels were elevated (40.2 pmol/L), and thyroid stimulating hormone (TSH) was low (0.1 uIU/mL). On readmission, fT4 levels were normal (11.8 pmol/L) while TSH remained low (0.005 uIU/mL).
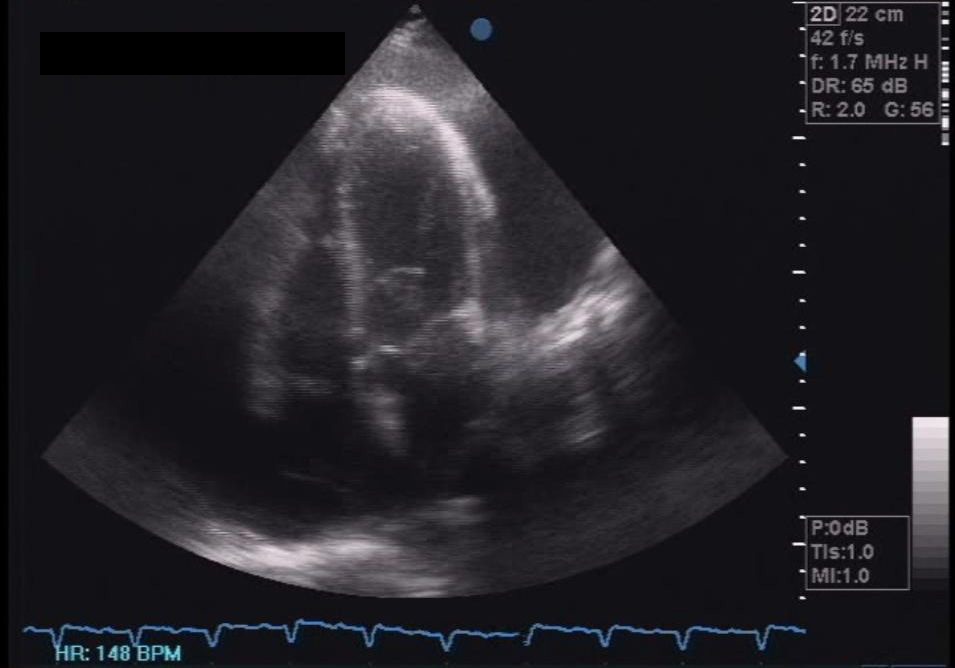
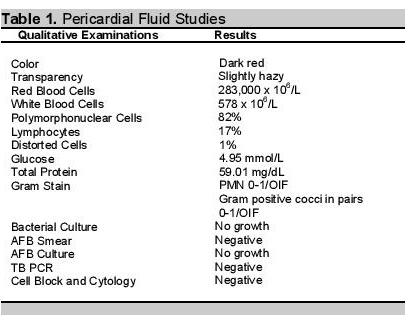
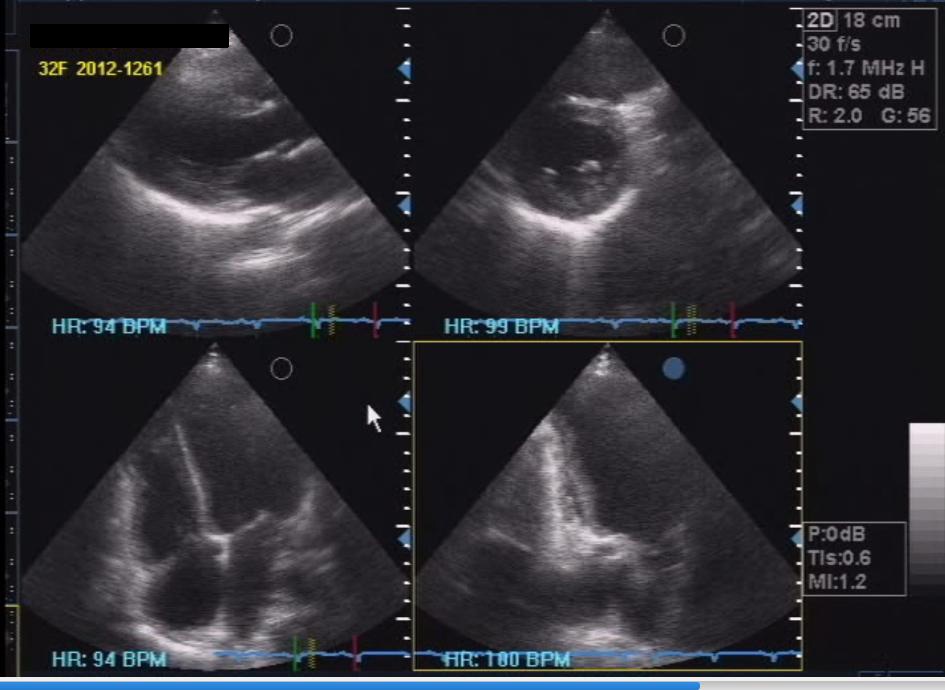
Her 12-lead electrocardiogram showed atrial fibrillation in rapid ventricular response, with nonspecific ST-T wave changes. A 2D echocardiogram with color-flow Doppler during the second admission showed normal-sized chambers with left ventricular remodelling and mildly depressed left ventricular systolic function; adequate right ventricular systolic function; mild to moderate pulmonary hypertension; and massive pericardial effusion in impending tamponade (Figure 3). Pericardial fluid samples were sent for differential cell counts, cytology, acid-fast (AFB) and conventional gram stain and culture, tuberculous polymerase chain reaction (TB PCR) and determination of glucose and total protein levels. Table 1 summarizes the pericardial fluid analysis.
Click here to download Figure 3Figure 3. Initial 2D echocardiogram showing circumferential pericardial effusion with suspicious right ventricular collapse during late diastole, a sign of tamponade.
Click here to download Table 1
Table 1.Pericardial Fluid Studies.
Fetal screening with congenital anomaly scan showed no gross fetal structural abnormalities, with an estimated sonographic weight that was appropriate for gestational age. Serum antinuclear antibody (ANA) to rule out the possibility of an autoimmune process was likewise negative.
The patient underwent pericardiocentesis with low flow drainage while on dobutamine infusion, draining around 600cc of serosanguinous fluid. Dobutamine was discontinued after the procedure, and the patient's vital signs remained stable throughout admission except for intermittent episodes of tachycardia. Medical therapy included methimazole, digoxin, prednisone and furosemide. Propranolol was also initiated after initial diuresis to control the heart rate and decrease the adrenergic effects of the hyperthyroid state. The patient experienced gradual resolution of orthopnea, pedal edema and exertional dyspnea, with spontaneous conversion to normal sinus rhythm. On the 18th hospital day, she was subsequently discharged.
Two months after discharge, the patient underwent a repeat 2D echocardiogram (Figure 4) revealing the absence of the pericardial effusion but with pericardial thickening. There was also improvement of overall left ventricular systolic function after treatment with heart failure and anti-thyroid drugs. The patient went into preterm labor at 32 weeks of gestation. She delivered a live baby girl via spontaneous vaginal delivery, who was admitted to the neonatal intensive care unit and eventually discharged.
Click here to download Figure 4Figure 4. Repeat 2D echocardiogram after two months, showing absence of pericardial effusion but with thickened pericardium.
Our patient initially presented with classic thyrotoxic symptoms associated with diffuse goiter, typical eye signs, pretibial dermopathy and acropachy. These findings, plus a high fT4 and low TSH, make the clinical diagnosis of Graves' disease straightforward.2 In thyrotoxic patients, the most common cardiac complications are sinus tachycardia, atrial fibrillation and congestive heart failure,1,3,4 with reports of heart block5 and a cardiomyopathy that may or may not be reversible following return to a euthyroid state.6,7 These manifestations may be exaggerated in pregnancy owing to the physiologic increase in heart rate, stroke volume and cardiac output.8
In thyroid disorders, pericardial effusion is more commonly seen in 5-30 % of hypothyroid patients.9 In contrast, its occurrence in Graves' disease is somewhat rare;10-14 one study looking into the causes of pericardial effusion in 204 patients found no cases of thyrotoxicosis among them.15 This lack of published evidence linking pericardial effusion with thyrotoxicosis emphasizes the importance of a high index of suspicion to ascertain the association between the two disease entities.10
Our initial consideration for the patient's effusion was an infectious etiology, specifically Mycobacterium tuberculosis, which is highly prevalent in the Philippines. Given that the patient is a female of reproductive age, the possibility of autoimmune pericarditis secondary to an underlying connective tissue disease such as systemic lupus erythematosus was also entertained.16 Malignancy was considered since it was found to be the most common etiology in a study among Filipino patients with pericardial effusion in tamponade.17 We also regarded the possibility that the effusion may have been due to the pregnancy itself, but it is usually mild and characteristically manifests as a transudate.18 Finally, the effusion may have been part of an unusual vasculitic side-effect from PTU; however, this was also unlikely as the other typical vasculitic manifestations of rash, hemoptysis, cytopenia, hematuria and renal failure were absent in the patient.19 Since workup for all these causes eventually turned out unremarkable (the gram stain result was most likely a contaminant), with the effusion resolving after pericardiostomy and anti-thyroid drugs (making an idiopathic cause less likely), we concluded that thyrotoxicosis caused the patient's effusion.
It is interesting that the patient's effusion was detected during readmission when she had already received anti-thyroid drugs and fT4 levels had normalized. This is consistent with reports showing that pericardial effusion resulting in tamponade can still develop in Graves' thyrotoxicosis even during anti-thyroid treatment and with improving thyroid function tests.11 Moreover, although the patient was initially discharged improved, the fact that some of her hyperthyroid symptoms (tachycardia, dyspnea, edema) persisted on readmission with TSH being suppressed further from 0.1 to 0.005 uIU/mL showed that the thyrotoxicosis was still not adequately controlled. PTU, initially started due to its generally better safety profile for pregnancy, was subsequently shifted to methimazole, a more potent antithyroid drug.
Since the apparent worsening of the patient's heart failure symptoms on readmission was temporally associated with her recent thyrotoxic manifestations, thyrotoxicosis was considered as the underlying cause of the heart failure, despite the initiation of standard cardiac medications. This is consistent with reports showing that in some patients treated for hyperthyroidism, achievement of a euthyroid state is not by itself sufficient to fully reverse cardiomyopathy and left ventricular failure.6,7,20 Furthermore, the patient's pregnant condition, persistent tachycardia, and atrial fibrillation could have also contributed independently to the exaggeration of the heart failure symptoms.8,21
The pathophysiology of thyrotoxic pericardial effusion has been demonstrated to be similar to that of pretibial myxedema, with a characteristic serous effusion attributed to the transudation of albumin and decreased lymphatic clearance of interstitial fluid proteins.22 On occasion, the effusion can also appear serosanguinous, as in our patient.11,12 This purported underlying pathophysiologic mechanism is the basis for several reports stating the potential benefit of prednisone in these patients.10,13 Hence, we decided to continue prednisone even after TB was ruled out as an unlikely etiology of the effusion.
Even with treatment, the mortality rate for thyrotoxicosis from cardiac sequelae can reach 30%.10 An echocardiogram is therefore recommended as part of the investigation to rule out pericardial effusion as a contributing factor potentially heralding the development of cardiac tamponade. Conversely, in a patient presenting with an unexplained pericardial effusion, it might be prudent to exclude Graves' thyrotoxicosis despite the absence of classic signs and symptoms, in addition to routine tests for microbiology, inflammatory and oncologic markers and autoimmunity.
This is the first documented case of its kind in the Philippines, and in the entire ASEAN region. Moreover, in contrast to other reports on thyrotoxicosis-related pericardial effusion, this is also so far the first case dealing with a pregnant patient. The rarity of the association emphasizes the need for vigilance.
1. Melmed S, Polonsky K, Larsen P, Kronenberg H, editors. Williams Textbook of Endocrinology, 12th edition. Philadelphia: Elsevier Saunders; 2011.
2. Baskin JH, Cobin RH, Duick DS, Gharib H, Guttler RB, Kaplan MM, et al. American Association of Clinical Endocrinologists Medical Guidelines for Clinical Practice for the Evaluation and Treatment of Hyperthyroidism and Hypothyroidism. Endocrine Practice. 2002 Dec; 8(6): 457-469.
3. Klein I, Ojamaa K. Thyroid hormone and the cardiovascular system. N Engl J Med. 2001; 344:501-509. http://dx.doi.org/10.1056/NEJM200102153440707.
4. Kahaly G, Dillmann W. Thyroid Hormone Action in the Heart. Endocrine Reviews. 2005; 26(5):704-728. http://dx.doi.org/10.1210/er.2003-0033.
5. Sampana A, Jasul G. High Grade AV Block Complicating Hyperthyroidism: A Case Report. Phil J Int Med. 2010 Jul-Sep; 48(2):38-40.
6. Umpierrez GE, Challapalli S, Patterson C. Congestive heart failure due to reversible cardiomyopathy in patients with hyperthyroidism. Am J of Med Sc. 1995; 310(3):99-102.
7. Ebisawa K, Ikeda U, Murata M, Sekiguchi H, Nagai R, Yazaki Y et al. Irreversible cardiomyopathy due to thyrotoxicosis. Cardiology. 1994; 84:274-7.
8. Jameson JL, Weetman AP. Disorders of the Thyroid Gland. In: Braunwald E, Fauci AS, Kasper D, Hauser HL, Longo D, Jameson JL, editors. Harrison's Principles of Internal Medicine, 15th edition. New York: McGraw-Hill; 2001.
9. Spodick DH. Pericardial Diseases. In: Braunwald E, Zippes DP, Libby P, editors. Heart Disease, 6th edition. Philadelphia: WB Saunders; 2001.
10. Ovadia, S, Lysy y L, Zubkov T. Pericardial effusion as an expression of thyrotoxicosis. Tex Heart Inst J. 2007;34(1):88-90.
11. Teague E, O'Brien C, Campbell N. Pericardial effusion and tamponade complicating treated Graves' thyrotoxicosis. Ulster Med J. 2009;78(1)56-58.
12. Nakata A, Komiya R, Ieki Y, Yoshizawa H, Hirota S, Takazakura E. A patient with Graves' disease accompanied by bloody pericardial effusion. Internal Medicine. 2005;44(10):1064-1068.
13. Clarke NRA, Banning AP, Gwilt DJ, Scott AR. Pericardial disease associated with Graves' thyrotoxicosis. QJM: An International Journal of Medicine. 2002; 95:188-189.
14. Khalid Y, Sulaiman R, Zahir R, Baskar V, and Buch HN. An unusual complication in a patient with Graves' disease. New Zealand Med J. 2011; 124 (1341).
15. Levy PY, Corey R, Berger P, Habib G, Bonnet JL, Levy S, et al. Etiologic diagnosis of 204 pericardial effusions. Medicine (Baltimore). 2003; 82(6): 385-91.
16. Imazio M. Pericardial involvement in systemic inflammatory diseases. Heart. 2011; 97(22):1882-1892. http://dx.doi.org/10.1136/heartjnl-2011-300054.
17. Tumabiene KD, Chiong, L, Macapugay, L. Clinical Profile and Outcomes of Adult Patients with Echocardiographic Evidence of Cardiac Tamponade at the Philippine General Hospital: A Five-Year Study (The CAPTIVE-HEART Study). Unpublished.
18. Abduuabbar HS, Marzouki KM, Zawawi TH, Khan AS. Pericardial effusion in normal pregnant women. Acta Obstet Gynecol Scan. 1991; 70(4-5):291-294. http://dx.doi.org/10.3109/00016349109007874.
19. Nakamori Y, Tominaga T, Inoue Y, Shinohara K. Propylthiouracil (PTU)-induced vasculitis associated with antineutrophil antibody against myeloperoxidase (MPO-ANCA). Intern Med. 2003; 42(6):529-33.
20. Nowak M, Madrzak D, Kuźniar J, Grzywa M. Reversible thyrotoxic cardiomyopathy - report of three cases. Pol Arch Med Wewn. 2002 Oct; 108(4):979-82.
21. Roffi M, Cattaneo F, Topol E. Thyrotoxicosis and the cardiovascular system: subtle but serious effects. Cleveland Clinic J of Med. 2003; 70:57-63.
22. Parving HH, Hansen JM, Nielsen SL, Rossing N, Munck O, Lassen NA. Mechanisms of Edema Formation in Myxedema - Increased Protein Extravasation and Relatively Slow Lymphatic Drainage. N Engl J Med. 1979; 301(9):460-465. http://dx.doi.org/10.1056/NEJM197908303010902.
Authors are required to accomplish, sign and submit scanned copies of the JAFES Declaration that the article represents original material that is not being considered for publication or has not been published or accepted for publication elsewhere.
Consent forms, as appropriate, have been secured for the publication of information about patients; otherwise, authors declared that all means have been exhausted for securing such consent.
The authors have signed disclosures that there are no financial or other relationships that might lead to a conflict of interest. All authors are required to submit Authorship Certifications that the manuscript has been read and approved by all authors, and that the requirements for authorship have been met by each author.