Childhood obesity is a serious global public health problem which has been increasing since last two decades. In the Philippines, we are facing the challenge of childhood obesity, particularly in urban areas. The national prevalence of childhood overweight and obesity in the Philippines has started to show an upward trend from 1993 to 2005; based on the Facts and Figures 2005 published by the Philippine Food and Nutrition Research Institute (FNRI), the prevalence of overweight, using a cut-off point of BMI 85th percentile, has doubly increased from 2.4% to 4.8 % (1993 to 2005) among adolescents (11-19 years).[1]The national prevalence based on the seventh Nutritional Survey by FNRI did not show a significant change from 2005 to 2008 (4.6%). [2] A more recent local study (2009) reported a higher overall prevalence of overweight and obesity (21%) among adolescents in Metro Manila. [3] Those from upper socioeconomic status and studying in the private schools are at higher risk to be obese.[4] ,[5],[6]
Obese adolescents are at risk to develop metabolic syndrome (MetS), which is defined as having three out of five components: abdominal adiposity or central obesity, hypertension, hypertriglyceridemia, low level of high density lipoprotein-cholesterol (HDL) and hyperglycemia. [7],[8] Currently, there are different definitions of MetS in adolescents. In United States, the National Cholesterol Education Program-Adult Treatment Panel III (NCEP-ATP-III) criteria, [9] modified for children and adolescents, and various other definitions were used. The International Diabetes Federation (IDF) in 2007 published a definition of MetS in children and adolescents.[10]
Studies have shown that the prevalence of MetS ranged from 8.9% in obese children to as high as 50% in severely obese adolescents.[11] ,[12],[13] Overweight and obese adolescents were shown to develop cardiometabolic complications at a young age, and also premature death in adulthood.[14] ,[15],[16], [17] In the Philippines, a study (2002) showed that one third of obese Filipino children and adolescents seen in a pediatric endocrine clinic had elevated total cholesterol and triglyceride levels, as well as low HDL-cholesterol levels.[18] In another study (2000) of obese Filipino children with mean age of 10.8 years and BMI >30, the mean fasting insulin level was high at 34.8 uU/ml. [19]Chronic non-communicable diseases including diabetes, obesity, hypertension and heart diseases which were once thought to affect mostly developed countries, are now becoming the major health problems in Asia.12,[20] These diseases have the greatest impact in low- and middle-income countries, where about three-fourths of deaths occur. So far, there has had no reported prevalence of MetS in Filipino adolescents. It is important to determine the prevalence of MetS in overweight and obese adolescents who are at risk to develop cardiometabolic diseases; the findings may provide insights for health professionals and policy-makers about the extent of these problems in young population.
This study was done to determine the prevalence of MetS in overweight and obese Filipino adolescents based on the IDF definition. It also determined the prevalence of individual components of MetS, as well as hyperinsulinemia and insulin resistance.
This study included a total of 350 overweight and obese adolescents (aged 10 to 18 years, 206 males and 144 females) who were referred to pediatric endocrine clinics in Metro Manila, Philippines from 2008 to 2010. Participants were included in the study with consent and assent. Adolescents who had pregnancy, known systemic diseases or other endocrine problems like thyroid dysfunction, Cushing syndrome, or took medications that altered blood pressure, glucose, or lipid metabolism were excluded from the study. The sample size was determined to achieve a 95% confidence interval and 0.05 margin of error based on a 35% prevalence rate of metabolic syndrome in overweight children and adolescents. [21],[22]
Height was measured in an upright position using a stadiometer and weight was determined using a standard weighing scale. The body mass index (BMI) is the weight in kilograms divided by the square of the height in meters. Waist circumference was measured at the midpoint between the lowest rib and the iliac crest, as recommended by WHO guidelines.[23] Waist-height ratio is the waist circumference in centimeters divided by height in centimeters. Three seated blood pressure (BP) readings were obtained and the mean of these BP readings was used for statistical analyses. Blood was extracted for fasting blood glucose, lipid profiles and insulin determination.
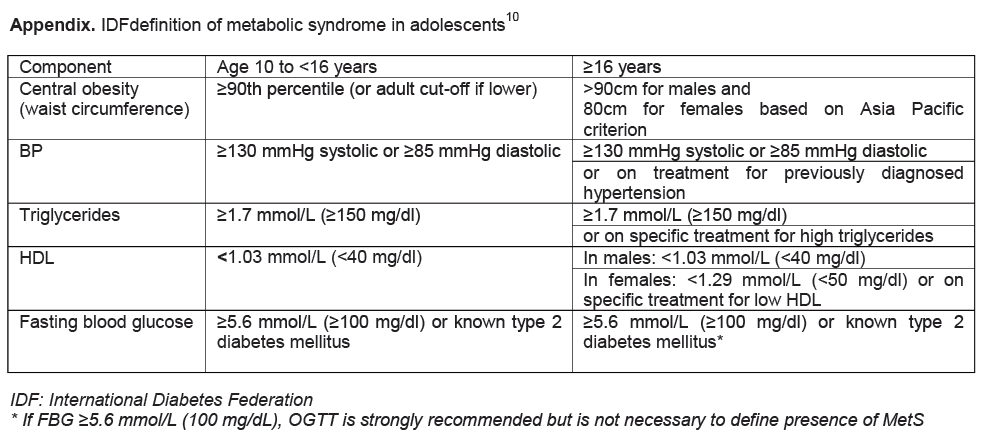
Based on the World Health Organization (WHO) reference, when a BMI z-score for age and gender is ≥+1 to <+2SD, it is classified as overweight; when a BMI z-score is ≥+2 SD, it is obesity.[24] Participants were considered as having MetS when they had at least three out of five clinical features: central obesity, hypertension, hypertriglyceridemia, low HDL level and hyperglycemia. In our study, the prevalence of MetS in the overweight and obese adolescents was determined based on the IDF definition10 (Appendix). Since there is no available national data of waist circumference in Filipino adolescents, abdominal adiposity or central obesity was based on a waist-height ratio of ≥0.5.[25] ,[26],[27], [28] Hyperinsulinemia was defined as fasting plasma insulin level >15 μU/ml.[29] Insulin resistance was determined based on a homeostasis model assessment (HOMA) using the following formula: Insulin Resistance Index (IR) = fasting serum insulin (mU/ml) multiplied by fasting plasma glucose (mmol/L) and divided by 22.5.[30] A HOMA-IR cut-off level of 3 in pediatric population was used to identify an insulin-resistance status.[31]
Descriptive statistics for continuous variables were expressed as the mean ± the standard deviation. Chi-square and Fisher Exact Probability Test were used for correlation of metabolic syndrome with individual components. Statistical significance was taken at p<0.05.
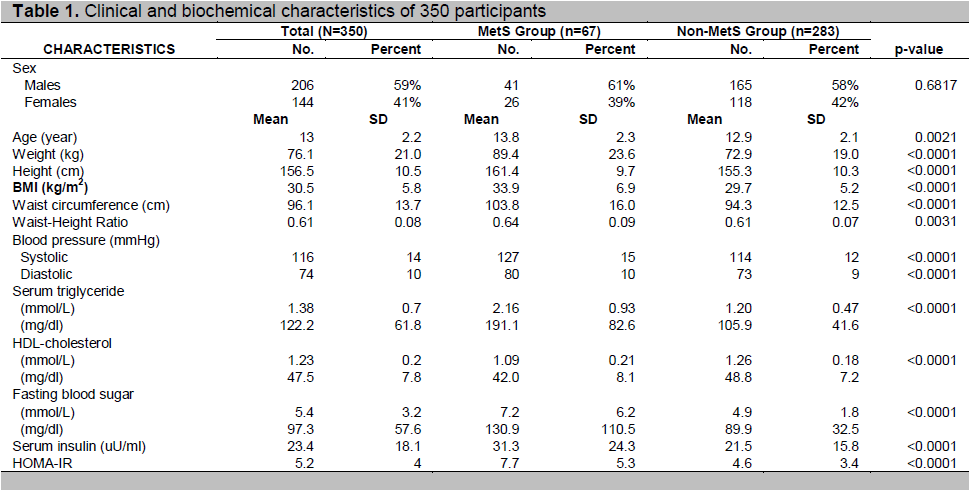
Among 350 participants, 206 (59%) were males and 144 (41%) females; 297 (85%) were obese and 53 (15%) were overweight. The age ranged from 10 to 18.8 years with a mean of 13 ±2.2 years. The BMI ranged from 20 to 51.7, with a mean of 30.5±5.8. There was no sex difference in most of the demographic and laboratory variables, except that males had larger waist circumference (p= 0.0004), waist-height ratio (p= 0.0009) and higher mean systolic BP (p= 0.0168).The clinical and biochemical characteristics of participants categorized by MetS were shown in Table 1.
According to the IDF definition, the overall prevalence of MetS in participants was 19% (67/350). Among obese adolescents, 21% (61/297) had MetS and among overweight adolescents, 11% (6/53) had MetS. In males, the prevalence of MetS was 20% (41/206) and in females, it was 18% (26/144); there was no significant sex predilection (p=0.6817) (Table 1). In MetS group, participants were heavier and taller with greater BMI, waist circumference, waist-height ratio, BP, triglyceride, fasting blood glucose (FBG), insulin and HOMA-IR levels, but lower HDL levels.
Click here to download Table 1
Table 1. Clinical and biochemical characteristics of 350 participants
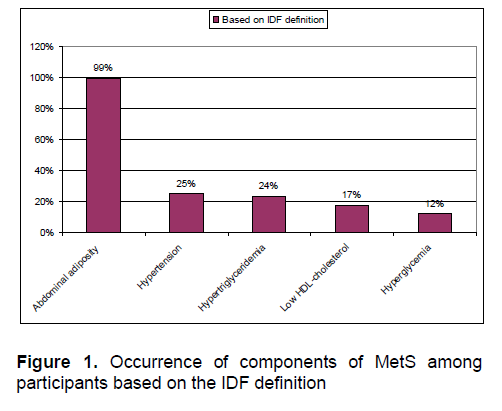
Based on the IDF definition, 14% of overweight and obese participants had 3 components, 5% had 4 components and none had 5 components. The remaining 81% participants had either 1 (46%) or 2 components (35%). The top 3 components of MetS were abdominal adiposity or central obesity, hypertension, hypertriglyceridemia, followed by low HDL and hyperglycemia. (Figure 1). There was no sex predilection in the prevalence of individual components (p >0.1).
Click here to download Figure 1
Figure 1. Occurrence of components of MetS among participants based on the IDF definition
Hyperinsulinemia and insulin resistance
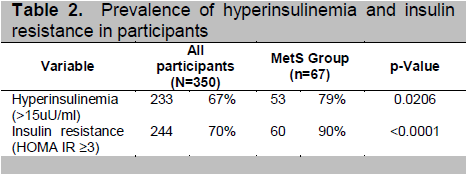
Among overweight and obese adolescents, 67% (233/350) had hyperinsulinemia (>15 uU/ml) and 70% (244/350) had insulin resistance based on HOMA-IR (≥3). Among those with MetS, the prevalence of hyperinsulinemia (79%) and insulin resistance (90%) was significantly higher (Table 2); the mean levels of serum insulin (31.3μU/ml) and HOMA-IR (7.7) were also high.(Table 1).
Click here to download Table2
Table 2. Prevalence of hyperinsulinemia and insulin resistance in participants
The rise in childhood obesity has contributed to the rising prevalence of the pediatric metabolic syndrome in developing countries.12 Using the IDF definition, the prevalence of MetS in Filipino overweight and obese adolescents (10 to <19 years) was 19%; it was higher than the reported 8.9% in both Portuguese (10-20 years, median age 13.4 years)[xxxii] and French (10 to <16 years) overweight and obese adolescents,[33] and also higher than 16.9% in obese Thai children and adolescents, [34] but lower than 33.2% among overweight and obese adolescents (up to age 16 years) of multi-ethnic origin in Netherlands, [35] 23.3% and 40.4% among the extremely obese adolescents (12-18 years) in Italy and Germany, respectively.[36]
The risk of MetS increased as weight became heavier. In our study, the prevalence of MetS was higher in the obese than overweight adolescents. Similar finding was noted in other studies, including an Indian adolescent population.22
There was no consistent sex predilection noted in the studies of MetS in adolescents. Our study showed the prevalence of MetS was higher in males but the difference was not statistically significant. In a study of obese Bolivian children and adolescents, MetS was also found in larger proportion among males (40%) than females (32.2%), but not statistically significant (p-- 0.599).[37] In obese Spanish pediatric population and Turkish obese adolescents, there were no differences by sex.[38] ,[39]
In this study, participants with MetS mostly had 3 components (14%), followed by 4 components (5%). Among extremely obese European adolescents, higher proportion of 4 components was reported: 16% of Italian cohort and 28% of German cohort.36 Top 3 components of MetS in our study were abdominal adiposity, hypertriglyceridemia and hypertension; hyperglycemia had the lowest prevalence. Such findings were similar to the Portuguese study which reported that the waist circumference was the most prevalent component and hyperglycemia the least.32 Another review also showed that hyperglycemia had the lowest prevalence but high triglyceride level was the most frequent component of the MetS. [40] The presence of cardiometabolic risks in obese adolescents should warrant early intervention to prevent further progression of complications.
Components of MetS included blood glucose but not insulin level. However, insulin resistance has been known as an important feature of MetS. The American Heart Association recommended adding fasting insulin determination to the evaluation of children at risk for insulin resistance. [41]
A longitudinal study demonstrated hyperinsulinemia in youth as a predictor of type 2 diabetes mellitus.
[42]In this study; fasting insulin and HOMA-IR levels were also determined. Although only 12% of participants had hyperglycemia, much more had hyperinsulinemia (67% ) and insulin resistance based on HOMA-IR (70%). Our study showed that the association between variables (hyperinsulinemia and insulin resistance) and MetS was statistically significant. Detection of hyperinsulinemia and insulin resistance should alert the clinician to do intervention to prevent the progression to overt diabetes mellitus as well as MetS.
Overweight and obese adolescents are at risk to develop MetS. The prevalence of hyperinsulinemia and insulin resistance is high.
This study was approved by the Ethics Review Board and received a research grant (NIH 2008-01-30-02) from the National Institute of Health, and Dr. Artemio Jongco-Cosme Cagas in Pediatric Endocrinology and Metabolism Professorial Chair/ Faculty Grant. The author would like to acknowledge research assistants, Dr. Catherine Anne G. Pangilinan and Mrs. Gina Reyes, Professor Ma. Lourdes E. Amarillo and Ms. April Dyan R. Furia for the statistical assistance and the participants in the study.
Click here to download Appendix
Appendix. IDFdefinition of metabolic syndrome in adolescents10
[1] . Food and Nutrition Research Institute, Department of Science and Technology. Philippine Nutrition: Facts and Figures, 2005. Taguig City: FNRI-DOST, 2007.