Recent evidence from animal and human studies suggests that vitamin D, both cholecalciferol and calcitriol, may play a role in modifying the risk of diabetes.1 Some authors have reported that vitamin D stimulated the expression of insulin receptor, thereby enhancing insulin responsiveness in glucose transport, improving glycaemic control, insulin secretion and insulin resistance.1-3
However, studies on the mechanism and appropriate doses of vitamin D are still unclear and requires further investigation.4,5 Further in vivo studies are needed to address the effect and the effective dosages of vitamin D in human adipose tissue as well as its relevance in associated diseases.6
GLUT4 has a main role in glucose metabolism and the maintenance of glucose homeostasis in the body; its activation has become a therapeutic target in pharmacological intervention strategies to control diabetes.7 Adipose tissue is a major site of glucose metabolism and has a critical role in the maintenance of glucose homeostasis.8
This study reports the effects of cholecalciferol in the histopathology of adipose tissue in diabetic rats, particularly related to its diameter and expression of GLUT4. This study is expected to reinforce the role of vitamin D as adjunctive therapy in diabetes mellitus.
Twenty eight Wistar strain adult male rats (Rattus norvegicus) matching the inclusion criteria were acclimatized for 7 days. Combination of high fat diet (lard 22.8%) and intraperitoneal injection of 35 mg/kg streptozotocin (STZ) on day 14 were used to induce diabetes.9,10 Furthermore, seven days after STZ injection, the FBG from all the rats' tail vein blood were evaluated. They were defined as diabetic rats when the FBG >135 mg/dl.11,12
All diabetic rats (nineteen) were divided into one control (K) group and three treatment groups (X1, X2, X3). Control group was a group of diabetic rats given propylene glycol in volume of 1 ml/100 gram/body weight (b.w). Treatment groups were groups of diabetic rats given cholecalciferol with a dose of 6.25 μg/kg b.w (concentration 0.625 μg/ml) in X1 group, 12.5 μg/kg b.w (concentration 1.25 μg/ml) in X2 group, and 25 mg μg/kg b.w (concentration 2.5 μg/ml) in X3 group. Cholecalciferol was given in propylene glycol in volume of 1 ml/100 g b.w per orem, every day for 14 days, starting on the 21st day. Twenty-four hours after the last treatment, the rats were then fasted for 12 hours and anesthetized with intramuscular injection of ketamine HCl in a dose of 44-60 mg/kg b.w, then the FBG was measured from intracardiac blood. The rats were sacrificed by decapitation.13 Subcutaneous adipose tissue was taken through a 1x1 cm incision on the abdominal wall up to the subcutaneous layer. The tissue was fixed in neutral buffered formalin solution to be processed into histological preparations by Haematoxylin Eosin (H&E) and immunohistochemistry staining. FBG, adipocyte diameter and GLUT4 expression were analyzed by one-way ANOVA test (α = 0.05) and Least Significant Difference (LSD) for Multiple Comparison Procedure (MCP).
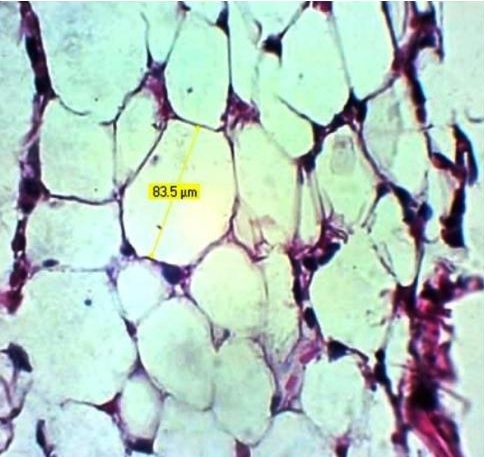
Click here to download Figure 1Figure 1.Photomicrograph of adipocyte diameter, H & E, X 400.
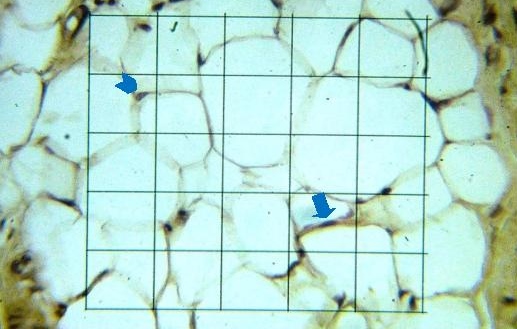
Click here to download Figure 2
Figure 2.Photomicrograph of GLUT4 expression in adipocyte, immunohistochemistry, graticulae, X 400. Arrow head: Adipocyte which did not express GLUT4, Arrow: Adipocyte which expressed GLUT4.
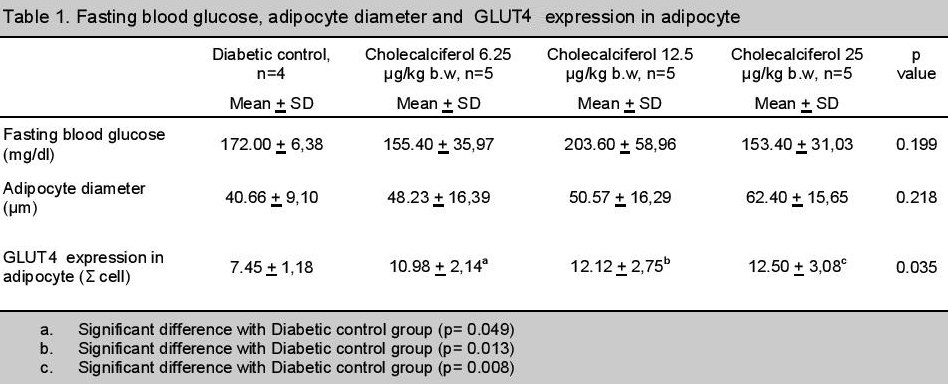
There was no significant difference in fasting blood glucose and adipocyte diameter between groups (p> 0.05). However, adipocyte diameter was increased in a dose dependent manner after administration of cholecalciferol.
There was significant difference in the expression of GLUT4 between groups (p <0.05). There was an increasing trend of GLUT4 expression along with the increased dose of cholecalciferol (Table 1).
Click here to download Table 1Table 1. Fasting blood glucose, adipocyte diameter and GLUT4 expression in adipocyte.
Our findings have shown that cholecalciferol administration could not improve blood glucose homeostasis nor increase adipocyte diameter in diabetic rats. These results were consistent with Calle, et al., (2008) who reported that the administration of calcitriol (1,25 dihydroxyvitamin D3) 3.75 μg/kg b.w intraperitoneally for 15 days did not correct hyperglycemia, glycosuria and could not increase adipocyte diameter in STZ-induced diabetic rats, but could normalize the number of insulin receptors in adipocytes.14 Another study by Anwar et al, (2013) reported that the subcutaneous administration of 10 μg/100g (0.1 μg/kg b.w) cholecalciferol for 6 days can reduce FBG by 26.31% in diabetic rats.2 These findings suggest that the route of administration may affect the effects of vitamin D in improving blood glucose levels and adipocyte diameter in diabetic rats.
Orwoll et al, (1994) reported that vitamin D has no effect on glucose homeostasis in uncontrolled diabetic patients.15 Another study reported that the administration of high dose cholecalciferol in type 2 diabetic patients was not related to improvement in glucose homeostasis but rather to improvement in plasma adiponectin levels.16 Recently, in vitro studies report that vitamin D may increase the surface area of inflammation-induced adipocyte, which is analogous to the diabetic condition.17 Thus, it can be concluded that vitamin D has more influences in inflammatory process mediated by adipokines produced by adipose tissue in diabetes.
The GLUT4 expressions increased dependently along with the increasing dose of cholecalciferol. However, this finding was not statistically significant. In line with the study by Manna & Jain (2012), vitamin D may increase the GLUT4 translocation and glucose utilization in adipocytes through activation of cystathionine-γ-lyase (CSE) and the formation of hydrogen disulfide (H2S).8
Cholecalciferol can increase the expression of GLUT4 in adipocyte without altering adipocyte diameter and FBG. It might be due to differences in tissue response to insulin. It has been understood that the response of GLUT4 to insulin in adipose tissue is higher than muscle tissue and the rate of fatty acid synthesis in adipocytes is strongly influenced by the plasma insulin concentration.18,19 However, in adipocytes of rats on a high fat diet, the fatty acid synthesis is highly unresponsive to insulin in which all lipogenic enzyme activities were decreased. A decreased intracellular capacity to utilize glucose for lipogenesis led to the decreased response of glucose metabolism to insulin in adipocytes of rats on high fat diet.20 In this study, although administration of cholecalciferol can increase adipocyte glucose uptake, it could not restore the intracellular capacity reduction in utilizing glucose for lipogenesis, which has been proven by non-significant increment in adipocyte diameter. Unfortunately, it has not been supported by the plasma insulin level as well as the rate of lipogenesis and lipolysis.
Adipose tissues only take up a small fraction of total body glucose uptake, but it increases along with the elevation of insulin level.21
In addition, administration of cholecalciferol in this study might be unable to inhibit glucose production in the liver and increase glycogen storages both in the liver and muscle tissue. There have been studies reporting that vitamin D administration can improve metabolic disorders in STZ-induced diabetic rats and provide therapeutic or protective effects for the liver, pancreas and kidneys of diabetic mice induced by alloxan.22,23 However, no study has reported the effects of vitamin D on metabolic disorders improvement in the liver of diabetic animals induced by a combination of high-fat diet and STZ. Although one study reported that vitamin D can increase GLUT4 translocation in the muscle tissue of STZ-induced diabetic mice, there is no study reporting the same finding in diabetic animal induced by combination method.24
Tannenbaum et al, (1997) reported that a high fat diet can lower glucose uptake in skeletal muscle and adipose tissue, decrease the number of insulin receptors in the liver, skeletal muscle and adipose tissue, decrease glycolysis and glycogen synthesis in the liver. High fat diet alters the activity of the hypothalamus-pituitary-adrenal in rats, thus increases the production of adrenal glucocorticoid. Increased adrenal glucocorticoid has antagonistic effects on insulin, leading to insulin insensitivity and decreased glucose uptake in insulin target tissues.25 Therefore, in this study, cholecalciferol could not significantly reduce FBG level, although the expression of GLUT4 in adipocyte was increased.
Cholecalciferol administration can increase adipocyte GLUT4 expression without altering fasting blood glucose level and adipocyte diameter in diabetic rats. Increasing the number of experimental animals, dose variations, duration of administration, and caloric restriction, may obtain a better outcome. Other methods, such as GLUT4 quantification in membrane fraction by Western blot may produce a more accurate result.
1. Pittas AG, Lau J, Hu FB and Dawson-Hughes B. The role of vitamin D and calcium in type 2 diabetes. A systematic review and meta-analysis. J Clin Endocrinol Metab. 2007;92(6):2017-2029. http://dx.doi.org/10.1210/jc.2007-0298.
2. Anwar MK, Hussain MH, Khan MA, Ahmad T. Effect of cholecalciferol and levo carnitine on plasma glucose, plasma insulin and insulin resistance in type 2 diabetic rats. J Pak Med Assoc. 2013;63(3):374-9. 3. Begoña M, Campion J, Dávila N, Calle C. Stimulation by 1, 25-dihydroxyvitamin D3 of insulin receptor expression and insulin responsiveness for glucose transport in U-937 human promonocytic cells. Endocrine Journal. 2000:47(4):383-391. http://doi.org/10.1507/endocrj.47.383 4. Danescu LG, Levy S, & Levy J. Vitamin D and diabetes mellitus. Endocr. 2009;35(1):11-17. http://dx.doi.org/10.1007/s12020-008-9115-5. 5. Seshadri KG, Tamilselvan B, Rajendran A. Role of vitamin D in diabetes. J Endocrinol Metab. 2011;1(2):47-56. http://dx.doi.org/10.4021/jem23w. 6. Mutt SJ, Hyppönen E, Saarnio J, Järvelin MR, Herzig, KH. Vitamin D and adipose tissue - more than storage. Front Physiol. 2014;5(228):1-9. http://dx.doi.org/10.3389/fphys.2014.00228. 7. Huang S, Czech MP. The GLUT4 glucose transporter. J Cell Metab. 2007;5(4):237-252. http://dx.doi.org/1-.1016/j.c.met.2007.03.006. 8. Manna P, Jain SK. Vitamin D upregulates glucose transporter 4 (GLUT4) translocation and glucose utilization mediated by cystathionine-γ-lyase (CSE) activation and H2S formation in 3T3L1 adipocytes. J Biol Chem. 2012;287(50):42324-32. http://dx.doi.org/10.1074/jbc.M112.407833. 9. Srinivasan K, Viswanad B, Asrat L, Kaul CL, Ramarao P. Combination of high-fat diet-fed and low-dose streptozotocin-treated rat: A model for type 2 diabetes and pharmacological screening. Pharmacological research. 2005;52(4):313-320. http://dx.doi.org/10.1016/j.phrs.2005.05.004. 10. Dewi AK, Sari DR. IL-2 level in diabetic mice due to obesity is higher than that in healthy mice. Folia Medica Indonesiana. 2013; 49(1):33-35. 11. Etuk EU. Animal models for studying diabetes mellitus. Agric Biol J N Am. 2010; 1(2):130-134. 12. Wang Z, Yang Y, Xiang X, Zhu Y, Men J, He M. Estimation of the normal range of blood glucose in rats. 2010;39(2):133-7, 142. 13. Smith JB, Mankoewidjojo S. Pemeliharaan, pembiakan dan penggunaan hewan percobaan di daerah tropis. Jakarta: UI Press. 1988;37-57. 14. Calle C, Begoña M, García-Arencibia M. Genomic actions of 1,25-dihydroxyvitamin D3 on insulin receptor gene expression, insulin receptor number and insulin activity in the kidney, liver and adipose tissue of streptozotocin-induced diabetic rats. BMC Molecular Biology. 2008;9(65):1-12. http://dx.doi.org/10.1186/1471-2199-9-65. 15. Orwoll E, Riddle M, Prince M. Effects of vitamin D on insulin and glucagon secretion in non-insulin-dependent diabetes mellitus. Am J Clin Nutr. 1994; 59(5):1083-7. 16. Breslavsky A, Frand J, Matas Z, Boaz M, Barnea Z, Shargorodsky M. Effect of high doses of vitamin D on arterial properties, adiponectin, leptin and glucose homeostasis in type 2 diabetic patients. Clin Nutr. 2013;32(6):970-975. http://dx.doi.org/10.1016/j.clnu.2013.01.020. 17. Zoico E, Franceschetti G, Chirumbolo S, et al. Phenotypic shift of adipocytes by cholecalciferol and 1a,25 dihydroxycholecalciferol in relation to inflammatory status and calcium content. Endocrinology. 2014 Nov;155(11):4178-88. http://dx.doi.org/10.1210/en.2013-1969. 18. Gould GW, Holman GD. The glucose transporter family: Structure, function and tissue-specific expression. Biochem. J. 1993;295(Pt 2):329-341. 19. Stansbie D, Brownsey RW, Cretitaz M, Denton RM. Acute effects in vivo of anti-insulin serum on rates of fatty acid synthesis and activities of acetyl-coenzyme a carboxylase and pyruvate dehydrogenase in liver and epididymal adipose tissue of fed rats. Biochem. J. 1976;160(2):413-416. 20. Lavau M, Fried SK, Susini C, Freychet P. Mechanism of insulin resistance in adipocytes of rats fed a high-fat diet. J Lipid Res. 1979;20(1):8-16. 21. James DE, Burleigh KM, Kraegen EW. Time dependence of insulin action in muscle and adipose tissue in the rat in vivo. An increasing response in adipose tissue with time. Diabetes. 1985;34(10):1049-1054. 22. George N, Kumar TP, Antony S, Jayanarayanan S, Paulose CS. Effect of vitamin D3 in reducing metabolic and oxidative stress in the liver of streptozotocin-induced diabetic rats. Brit J Nutr. 2012;108(8):1410-1418. http://dx.doi.org/10.1017/S0007114511006830. 23. Hamden K, Carreau S, Jamoussi K, et al. 1a,25 dyhidroxivitamin D3: Therapeutic and preventive effects against oxidative stress, hepatic, pancreatic, and renal injury in alloxan induced diabetes in rats. J Nutr Sci Vitaminol. 2009;55(3):215-222. http://doi.org/10.3177/jnsv.55.215. 24. Sakinah EN. Pharmacodynamics study of cholecalciferol to GLUT4 protein translocation in muscle fiber of hyperglycemia mice which inducted by streptozotocin. Folia Medica Indonesiana. 2013;49(3):134-138. 25. Tannenbaum BM, Brindley DN, Tannenbaum GS, Dallman MF, McArthurMD, Meaney MJ. High-fat feeding alters both basal and stress induced hypothalamic- pituitary-adrenal activity in the rat. Am J Physiol. 1997;273(6 Pt 1):E1168-77.Authors are required to accomplish, sign and submit scanned copies of the JAFES Declaration that the article represents original material that is not being considered for publication or has not been published or accepted for publication elsewhere.
Consent forms, as appropriate, have been secured for the publication of information about patients; otherwise, authors declared that all means have been exhausted for securing such consent.
The authors have signed disclosures that there are no financial or other relationships that might lead to a conflict of interest. All authors are required to submit Authorship Certifications that the manuscript has been read and approved by all authors, and that the requirements for authorship have been met by each author.