HIV/AIDS is a very important public health problem nowadays. HIV/AIDS pandemic not only leads to morbidity and mortality related to opportunistic infections, but also some forms of AIDS endocrinopathies (AIDS-related endocrine disorders).1,2 One of the endocrine disorders which is related to immunodeficiency state in HIV/AIDS and opportunistic infections such as tuberculosis, cytomegalovirus (CMV), and fungal infection is primary adrenal insufficiency known as Addison's disease.1-3 It can be said that hypoadrenalism is one complication that has been well-documented in patients with HIV/AIDS. Addison's disease results from bilateral destruction or dysfunction of the adrenal cortex marked by failure of the adrenal cortex to produce cortisol, aldosterone, and androgen.4 This disease has broad clinical features from mild to life-threatening conditions. The clinical features depend on the extent of loss of adrenal function and whether mineralocorticoid production is preserved.5
Opportunistic infections are known to cause adrenalitis and adrenal insufficiency in patients with AIDS.2 Several authors have published case reports and findings of CMV adrenalitis in patients with AIDS.3,6 Other microorganisms involving the adrenals in immunocompromised patients are Mycobacterium tuberculosis, Cryptococcus neoformans, Histoplasma capsulatum, Pneumocystis carinii, and Toxoplasma gondii.2 In rare conditions, the adrenals can also be affected by lymphoma and Kaposi’s sarcoma in patients with AIDS. Another cause of hypoadrenalism is the use of antifungal drugs for fungal opportunistic infections. Several drugs which can alter adrenal cortex hormone production are ketoconazole, megestrol acetate, rifampin, amphotericin B, trimethoprim, and sulphonamide.2
This is an interesting case of a man who was infected with HIV/AIDS and has been treated for multiple opportunistic infections. He experienced generalized hyperpigmentation and reduction in body hair for which he has been diagnosed as having primary adrenal insufficiency from clinical features and laboratory examinations. Treatment with longterm corticosteroids, clinical monitoring, and evaluation of the therapy is mandatory for glucocorticoid replacement, to reduce symptoms, and prevent life-threatening conditions.
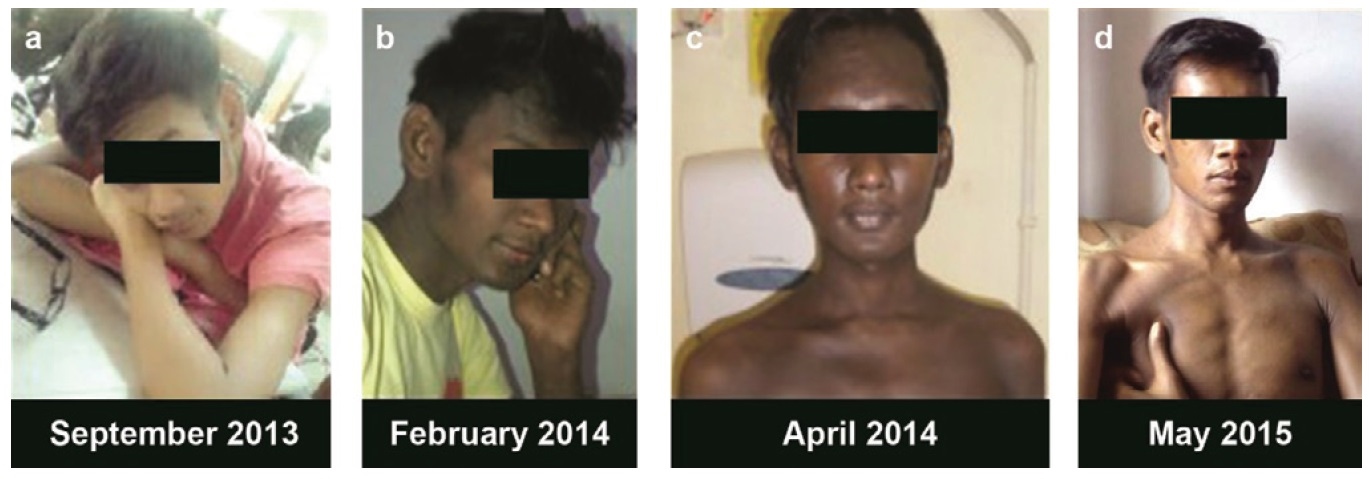
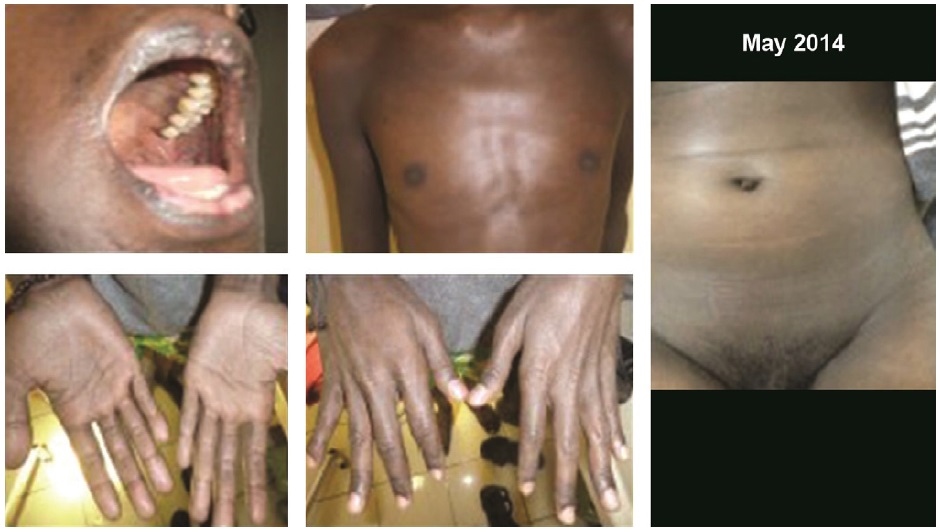
A 27-year-old man has been diagnosed with AIDS 6 months earlier with multiple opportunistic infections; cryptococcal meningitis, cytomegalovirus retinitis, and pulmonary tuberculosis, treated previously with amphotericin B, anti-tuberculosis drugs, and fluconazole. Later, he also got valganciclovir for cytomegalovirus infection. He reported darkening of the whole skin 2 months prior, accompanied by muscle weakness, chronic fatigue, reduced body weight, and thinning of body hair especially axillary and pubic hair (Figure 1). On physical examination, the whole body was darkened, even in the palmar hand, oral mucosa, along with reduced body hair (disappearance of axillary hair and thinning of pubic hair) (Figure 2).
Click here to download Figure 1Figure 1. Photographs of the patient: September 2013, February 2014, April 2014, May 2015.
Click here to download Figure 2
Figure 2. Photographs of the whole body showing hyperpigmentation of oral mucosa and palmar region, and thinning of the body hairs (axillary and pubic hair).
Routine laboratory examination revealed normal. Anti-HIV was reactive with CD4 count of 46. Morning cortisol was in the lower limit level at 5.6 μg/dL (normal: 4.3 to 22.4 μg/dL) while ACTH (adrenocorticotrophic hormone) level was extremely high at 90.2 pmol/L (normal: 2.2-13.3 pmol/L). The adrenal glands on abdominal CT scan were normal. Working together with a dermatopathologist, we did skin biopsy which showed hyperpigmentation caused by systemic disease (suggestive of Addison's disease).
Our patient was finally diagnosed as having primary adrenal insufficiency with differential diagnoses of HIV adrenalitis, opportunistic infection (CMV, TB, cryptoccocal)-related adrenalitis. Medication used in our patient was steroid (prednisone 7.5 mg/day divided into 5 mg in the morning and 2.5 mg in the afternoon following the circadian rhythm of cortisol), while HAART (highly active anti-retroviral therapy) which are tenofovir, lamivudine, efavirenz. Longterm regimen for opportunistic infections were likewise continued (valganciclovir, anti-tuberculosis drugs, and fluconazole).
After longterm therapy with prednisone, HAART, valganciclovir, anti-tuberculosis drugs, and fluconazole, there was clinical improvement of the patient; chronic fatigue and muscle weakness decreased, the skin became faintly lighter although still dominantly hyperpigmented (Figure 1d). In one year of HAART, CD4 count reached 196 and viral load was not detected. Eventually, valganciclovir, anti-tuberculosis drugs, and fluconazole have been stopped.
Primary hypoadrenalism is one of the well-documented manifestations of HIV/AIDS-related endocrine disorders.1,2 Our patient has multiple opportunistic infections which increase the risk of having disorder in his adrenal glands. In several case reports, it has been noted that either tuberculosis, CMV, or cryptococcal infection in adrenal gland can lead to adrenal insufficiency.3,6-8 Unfortunately, this patient had all of these opportunistic infections. He had been treated with anti-tuberculosis drugs, amphotericin B, and fluconazole. Later, he was also treated with valganciclovir for CMV retinitis.
His medications can lead to adrenal cortex disturbance inducing adrenal insufficiency. It has been reported in several studies and case reports that the use of azole groups to treat fungal infection is correlated with decrease of adrenal cortical hormones.9 Use of high-dose fluconazole has been reported to lead to adrenal insufficiency in critically ill patients.10,11 The basis for adrenal suppression by the azole antifungal agents is by suppression of the cytochrome P-450 enzyme system in the adrenal cells.9-12 In the culture of normal adrenals, fluconazole suppressed corticosterone, 17-hydroxypregnenolone, and androstenedione levels, whereas concentrations of progesterone, deoxycorticosterone, and 11-deoxycortisol increased.12
The clinical features of the patient are typical manifestations of Addison's disease.5 The generalized hyperpigmentation caused by increased production of pro-opiomelanocortin, a prohormone which is cleaved to ACTH and MSH-α (melanocyte-stimulating hormone-α) which accompanies the secretion of ACTH. ACTH and MSH-α are equally potent stimulators of melanogenesis. It is likely that the combination of increases in ACTH and MSH-α resulted to generalized hyperpigmentation in Addison's patient.5 Thinning of the body hairs especially axillary and pubic hairs are signs of hypoandrogenism in this patient. It has been published from long time ago the importance of the adrenal factor in the development of secondary sex characteristics.13
The patient has decreased morning cortisol level with increased ACTH level. This laboratory examination is matched with the typical finding of primary adrenal insufficiency.14 Actually, Addison's disease is a term wherein primary adrenal insufficiency is caused by the irreversible destruction or failure of adrenal cortex due to infection. Thomas Addison first described this disorder in patients with destruction of their adrenal glands caused by tuberculosis.5 Contrasting with the classic presence of hyponatremia and hyperkalemia, our patient didn’t develop hyponatremia and hyperkalemia, suggesting that mineralocorticoid is less disturbed than glucocorticoid and androgen.5,14
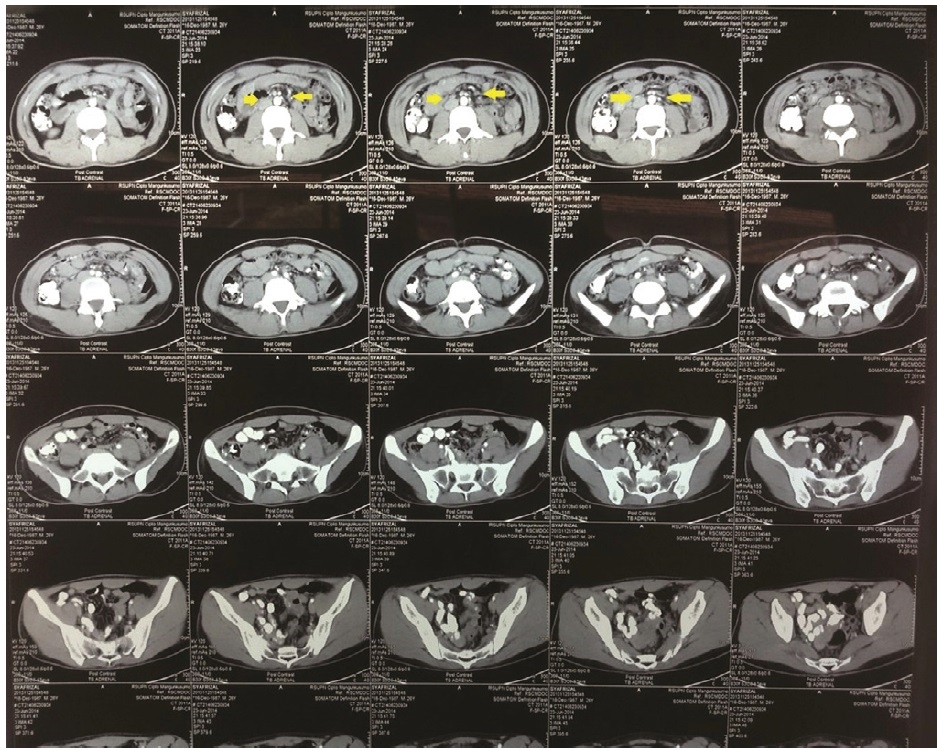
The abdominal computed tomography scan failed to show any gross adrenal pathology. This phenomenon suggested that the adrenalitis is not captured by the radiologic examination, and is unlikely caused by tuberculosis which can be seen in adrenal CT scan (Figure 3).7 In this situation, biopsy and culture of the adrenal gland is the gold standard to reveal the definitive and etiologic diagnosis of the primary adrenal insufficiency. But, adrenal biopsy is not routinely done in patients with classic clear clinical presentation and matched laboratory examination. Adrenal biopsy is done usually in postmortem examination to study the cause of primary adrenal insufficiency.6
Click here to download Figure 3Figure 3. Adrenal CT scan of the patient showing normal adrenals.
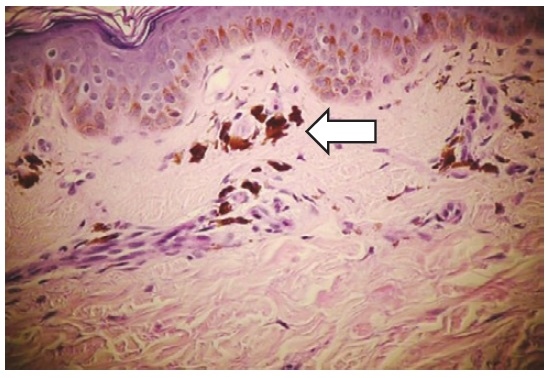
In this patient, we did skin biopsy because at the beginning of skin darkening, our multidisciplinary team included a dermatologist who suspected drug eruptions as the etiology of the skin changes of the patient. We found that the hyperpigmented skin is cause by melanin pigment excess in the epidermis layer as seen in the histopathology slides (Figure 4). At routine clinical practice, skin biopsy is not necessary for patients with Addison's disease, except that there are other conditions where histopathology examination of the skin is needed such as in uncommon presentation of drug eruption or skin malignancy.
Click here to download Figure 4Figure 4. Skin biopsy slide of the patient showing excess of melanin pigment.
Primary adrenal insufficiency is considered to be an incurable disease with a need for lifelong glucocorticoid (and mineralocorticoid) replacement therapy.15 In adrenal insufficiency, DHEA secretion is also decreased resulting to hypoandrogenism. In Europe, review from Grossman A et al15 summarizes general therapies used for adrenal insufficiency. Choices of glucocorticoid agent are hydrocortisone, cortisone acetate, prednisolone, and dexamethasone, while for mineralocorticoid, it is common to use 9-α-fludrocortisone. DHEA is a precursor for androgen, but not regarded as standard replacement regimen for adrenal insufficiency patient. Our patient was prescribed with prednisone which is a pro-drug converted via hepatic metabolism to prednisolone. It was given in divided dosage in the morning and afternoon following the circadian rhythm of cortisol. HAART and treatment for opportunistic infections were continued.
Primary adrenal insufficiency is an AIDS-related endocrinopathy which has a special clinical characteristic marked as darkening of the whole skin (generalized), accompanied by muscle weakness, chronic fatigue, and reduced body weight. Some patients show mineralocorticoid deficiency which manifests with hypokalemia, hyponatremia, and hypoglycemia. This condition can be caused by tuberculosis, CMV, cryptococcal, or HIV-related adrenalitis, and also antifungal therapy commonly used in HIV/AIDS patients. Our patient was finally diagnosed as having primary adrenal insufficiency (Addison's disease) and treated with longterm glucocorticoid replacement therapy using prednisone, while HAART and regimens for opportunistic infections were continued.
Ethical Clearance
The patient in this case report has given his permission to publish his case and use his photographs for this case report. The patient also attended the case meeting consisting of internist-endocrinologist, internist-allergologist, clinical immunologist, dermatologist, and dermatopathologist where his medical problems were discussed.
We would like to thank Fifinella Raissa, MD (Dermatologist) from the Department of Dermatovenereology, Faculty of Medicine University of Indonesia/Cipto Mangunkusumo Hospital for doing skin biopsy for the patient and also for the histopathology slide used in this article.
1. Tripathy SK, Agrawala RK, Baliarsinha AK, et al. Endocrine alterations in HIV-infected patients. Indian J Endocrinol Metab. 2015;19(1):143-7. http://dx.doi.org/10.4103/2230-8210.146870.
2. Grunfeld C, Lee G. AIDS endocrinopathies. In: Gardner DG, Shoback D, eds. Greenspan’s Basic and Clinical Endocrinology. 8th edition. San Fransisco: McGrawHill Companies, ch. 25, pp. 894-908, 2007. 3. Fujii K, Morimoto I, Wake A, et al. Adrenal insufficiency in a patient with Acquired Immunodeficiency Syndrome. Endocr J. 1994;41(1):13-8. http://dx.doi.org/10.1507/endocrj.41.13. 4. Betterle C, Scarpa R, Garelli S, Morlin L, Lassarotto F, Presotto F, et al. Addison's disease: A survey on 633 patients in Padova. Eur J Endocrinol. 2013;169:773-84. http://dx.doi.org/10.1530/EJE-13-0528. 5. Raff H, Sharma ST, Nieman LK. Physiological basis for the etiology, diagnosis, and treatment of adrenal disorders: Cushing’s syndrome, adrenal insufficiency, and congenital adrenal hyperplasia. Compr Physiol. 2014;4(2):739-69. http://dx.doi.org/10.1002/cphy.c130035. 6. Takasawa A, Morimoto I, Wake A, et al. Autopsy findings of Addison's disease caused by systemic cytomegalovirus infection in a patient with Acquired Immunodeficiency Syndrome. Intern Med. 1995;34(6):533-6. http://dx.doi.org/10.2169/internalmedicine.34.533. 7. Patnaik MM, Deshpande AK. Diagnosis- Addison's disease secondary to tuberculosis of the adrenal glands. Clin Med Res. 2008;6(1):29. http://dx.doi.org/10.3121/cmr.2007.754a. 8. Hung ZS, Lai YH, Hsu YH, Wang CH, Fang TC, Hsu BG. Disseminated cryptococcosis causes adrenal insufficiency in an immunocompetent individual. Intern Med. 2010;49(11):1023-6. http://dx.doi.org/10.2169/internalmedicine.49.3051. 9. Gradon JD, Sepkowitz DV. Fluconazole-associated acute adrenal insufficiency. Postgrad Med J. 1991;67(794):1084-5. 10. Albert SG, DeLeon MJ, Silverberg AB. Possible association between high-dose fluconazole and adrenal insufficiency in critically ill patients. Crit Care Med. 2011;29(3):668-70. 11. Krishnan SGS, Cobbs RK. Reversible acute adrenal insufficiency caused by fluconazole in a critically ill patient. Postgrad Med J. 2006;82(971):e23. http://dx.doi.org/10.1136/pgmj.2006.047258. 12. Van der Pas R, Hofland LJ, Hofland J, Taylor AE, Arlt W, Steenbergen J, et al. Fluconazole inhibits human adrenocortical steroidogenesis in vitro. J Endocrinol. 2012;215:403-12. http://dx.doi.org/10.1530/JOE-12-0310. 13. Mussio Fournier JC, Pollack E, Lussich Siri JJ. Loss of axillary and pubic hair in a patient ith Addison’s disease and regular menstruation: A case report. J Clin Endocrinol Metab. 1949;9(6):555. http://dx.doi.org/10.1210/jcem-9-6-555. 14. Michels A, Michels N. Addison disease: Early detection and treatment principles. Am Fam Physician. 2014;89(7):563-8. 15. Grossman A, Johannsson G, Quinkler M, Zelissen P. Therapy of Endocrine Disease: Perspectives on the management of adrenal insufficiency: Clinical insights from across Europe. Eur J Endocrinol. 2013;169:R165-75. http://dx.doi.org/10.1530/EJE-13-0450.Authors are required to accomplish, sign and submit scanned copies of the JAFES Declaration that the article represents original material that is not being considered for publication or has not been published or accepted for publication elsewhere.
Consent forms, as appropriate, have been secured for the publication of information about patients; otherwise, authors declared that all means have been exhausted for securing such consent.
The authors have signed disclosures that there are no financial or other relationships that might lead to a conflict of interest. All authors are required to submit Authorship Certifications that the manuscript has been read and approved by all authors, and that the requirements for authorship have been met by each author.