Manju Chandran, MD
Consultant and Director, Osteoporosis and Bone Metabolism Unit
Department of Endocrinology
Singapore General Hospital
President-Endocrine and Metabolic Society of Singapore
Outram Road, Singapore 169608
Tel. No.: (65)63214654
Email: manju.chandran@sgh.com.sg
e-ISSN 2308-118x
Printed in the Philippines
Copyright © 2012 by the JAFES
Received September 27, 2012. Accepted October 29, 2012.
Osteoporosis is a major public health problem worldwide and it is of particular significance in a rapidly aging society such as Singapore. In order to prevent the devastating sequelae of osteoporosis, it is important, to diagnose the condition early, to identify its associated risk factors and to initiate pharmacotherapy promptly when required. In addition, it is important to increase awareness of this condition amongst both health care professionals and the public. The Singapore Ministry of Health (MOH) Clinical Practice Guidelines published in 2008 was an update of a previously published version in 2002. It aimed at providing the best evidence-based recommendations for diagnosis, classification, evaluation and multidisciplinary management of osteoporosis and osteoporotic fractures in Singapore. This article summarizes the guidelines, discusses their applicability and utilization in Singapore and briefly outlines updates in management since the publication of the CPG.
Keywords: Osteoporosis, Clinical Practice Guidelines, Asia, Singapore, Secondary Fracture Prevention, Secondary Osteoporosis
The Singapore (MOH) Ministry of Health Clinical Practice Guidelines (CPG) for osteoporosis was published in March 2008. The guidelines, based on the best available evidence that was available at that time point provided practical recommendations relevant to the local context. The guidelines were developed to assist both general practitioners as well as specialists in managing osteoporosis, a disease which is of particular significance in an ageing society like Singapore. The guidelines were meant not to be viewed as a protocol, but instead were intended to provide an evidence based point of reference that could be used by doctors in their daily practice.
The stated objectives of the Singapore MOH CPG were to provide a framework to assist both general practitioners as well as specialists in the diagnosis and management of osteoporosis wi thout restricting the physician’s individual judgment, to provide a review of the therapeutic agents available for the treatment of osteoporosis with the aim of reducing fracture rates, to aid primary care physicians to decide when to refer patients with difficult problems to the relevant specialists and to highlight some areas where further research may be pursued. The guidelines were developed by a committee of general practitioners, endocrinologists, geriatricians, rheumatologists, gynaecologists, orthopaedic surgeons, physiotherapists, dieticians, and patient representatives appointed by the Ministry of Health. They were developed using the best of evidence available at that time. The levels of evidence were given ratings from 1++ to 4 and recommendations were given grades of A to D or a GPP (Good Practice Points) grade (Table 1).
This article summarizes the guidelines of 2008 and indicates the levels of evidence upon which each recommendation was made and the grades of each recommendation. It discusses their utility and implementation in Singapore and briefly outlines new developments in the diagnosis and management of osteoporosis since the publication of the CPG.
|
|
|
Doc Mads, please advise po. Sa style sheet natin hanggang 2nd subheading lang ang covered. And paki-advise na din po for the rest of the subheadings, parang nalito na ata yung author, pati yung conclusion kasi naging same format as the 3rd subheadings. |
Singapore has a rapidly ageing population and by the year 2030, it is estimated that one in five residents will be 65 years or older1. In Singapore, the incidence of hip fractures has increased 1.5 times in men and 5 times in women since the 1960’s2,3. This worrisome trend is expected to continue in the coming years. Age adjusted rates for hip fractures among Singaporean women are currently amongst the highest in Asia and approaching those of the west.3
According to the WHO, osteoporosis is defined as a progressive systemic skeletal disease characterised by low bone mass and micro-architectural deterioration of bone tissue, with a consequent increase in bone fragility and susceptibility to fracture. The National Institutes of Health (NIH), U.S.A (2000) consensus conference modified this definition as follows: “a skeletal disorder characterized by compromised bone strength predisposing a person to an increased risk of fracture. Bone strength reflects the integration of 2 main features:bone density and bone quality.” In the absence of methods of measuring bone quality, the diagnosis of osteoporosis tends to be made on the basis of low bone density. Osteoporosis can also be defined clinically on the basis of the presence of a fragility fracture. A fragility fracture is one that occurs as a result of minimal trauma such as a fall from a standing height or less, or no identifiable trauma.
Click here to download Table 1
Table 1. Types of Evidence and Grades of Recommendation

Bone mass is usually measured as bone mineral density (BMD) and prospective trials have shown that the risk of fracture increases progressively with decreasing BMD. Diagnostic thresholds based on the number of standard deviations (SD) above or below the peak bone mass of young adults (T-score), have been used to define categories of bone mass, as per the WHO classification.
It was recommended by the CPG that Dual Energy X-Ray Absorptiometry should be the method of choice for assessment of BMD (Grade A, Level 1+). The CPG opinioned that bone mineral density measurements using dual energy X-ray absorptiometry should be performed by trained dedicated staff, with appropriate quality control measures to ensure reliable results and that hip and spine DXA measurements should be measured and expressed as T and Z-scores based on Singaporean reference databases 4,5 [Grade C, Level 2+]. Quantitative USS was not recommended as a method for BMD assessment in the CPG 2008 since the workgroup found that there was a lack of validated threshold levels for heel USS for diagnosis of osteoporosis or for monitoring response to therapy [GPP].
The CPG recommended that evaluation should be targeted towards identifying and excluding modifiable causes of low bone mass like chronic steroid usage [GPP]. A non-exhaustive list of risk factors for osteoporosis and fractures and causes of secondary osteoporosis is provided in the CPG (Tables 3a and 3b). It was recommended that bone mass independent risk factors as listed below, should be assessed and managed appropriately. Individuals with osteoporosis should have relevant laboratory and radiological assessments to exclude diseases that may mimic, cause or aggravate osteoporosis.
Click here to download Table2a
Table 2a. Risk factors for osteoporosis, falls and fractures

Click here to download Table 2b
Table 2b. Causes of secondary osteoporosis

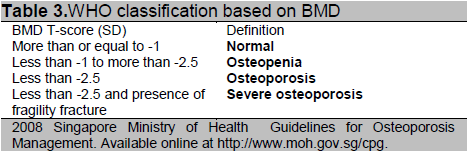
The Osteoporosis Self-Assessment Tool for Asians (OSTA) is a simple tool (see Table 4) based on age and weight which has been developed for the assessment of postmenopausal Asian women 6. The index derived from the tool is able to categorize women into high, moderate and low risk of being diagnosed with osteoporosis on subsequent bone mineral density (BMD) measurement. Women identified as high risk using the OSTA, should be advised to undergo bone mineral density measurement. A case-finding approach should be employed for women falling into the moderate risk category and they should be evaluated for clinical risk factors (see Table 2), and have BMD measured if clinical risk factors are present. The prevalence of osteoporosis is low enough in the low risk category for bone mineral density to be deferred, unless the woman has other identified clinical risk factors [Grade C, Level 2+].
Click here to download Table 3
Table 3. WHO classification based on BMD
Click here to download Table 4
Table 4. OSTA
1) Calcium
Low calcium intake (less than 250 to 500 mg/day) has been associated with lower bone density7 and higher risk of fracture8 in Asian populations. Higher dietary calcium intake was associated with lower risk of fracture9, though there is little data to support consumption of calcium in excess of 2000 mg/day. The CPG agreed with recommendations from the Singapore Health Promotion Board that adult Singaporeans should consume 800 to 1000 mg/day of calcium from dietary and/or calcium supplementation.
2) Vitamin D
A meta-analysis10 has shown that vitamin D supplementation between 700 to 800 units/day reduces the risk of hip and non-vertebral fractures in institutionalised and ambulatory elderly persons, whereas 400 units/day was not shown to be sufficient. Another meta-analysis11 also showed that vitamin D reduced the risk of falling in elderly (mean age > 70 yrs) individuals. The CPG suggested that vitamin D supplementation (with calcium) should be considered in most individuals, particularly in the elderly and institutionalised [Grade B, Level 1+]. Dosage of vitamin D to be used was not specified in the CPG, though caution was advised to avoid hypercalcemia when prescribing calcium and vitamin D in combination.
3) Exercise
Evidence exists to show that exercise in women with osteoporosis improves muscle strength, postural stability and bone mineral density12,13. This formed the basis for the recommendation by the CPG for specific exercise training programs in the management of osteoporosis [Grade B, Level 1+].
Types of exercises that were recommended in the CPG include:
a) Resistance exercise, either free weights or weight machines, at an intensity of 70 to 80% of maximum heart rate and 10 to 15 repetitions at low to moderate weight
b) Weight bearing (impact) exercise like aerobics, brisk walking, jogging, skipping and dancing at an intensity of 50 to 70% of maximal heart rate
The CPG recommended exercise at least 2 to 3 times per week, each lasting about 50 to 60 minutes which would include 10 minutes warm up, 20 minutes impact, 15 minutes resistance and 10 minutes cool down.
4) Cigarette smoking and excessive alcohol consumption
Both cigarette smoking14 and excessive alcohol15 intake are associated with increased risk of osteoporotic fractures and hence it was recommended that patients should be counselled on smoking cessation and limiting alcohol consumption [Grade C, Level 2+].
5) Use of hip protectors for the prevention of hip fractures in older people
CPG suggested that hip protectors16,17 may be used in people with high risk of hip fracture, in particular nursing home residents [Grade B, Level 1+].
6) Prevention of falls
CPG recommended that older patients should have an assessment on risk factors for falls and be managed with an individualised multi-factorial intervention which may include medication adjustments, home environment hazard assessment and modification, vision correction, physical therapy and addressing reversible medical conditions 8,19 [Grade C, Level 2+).
The CPG recommended that the decision to treat should not merely be based on BMD criteria. Factors to consider in the decision making would include those that are listed in Table 5 in addition to risk factors assessment for osteoporosis, falls and fractures [Grade C, Level 2+].
Click here to download Table 5
Table 5. Factors to consider in decision to treat
At the time of publication in 2008, the CPG acknowledged the presence of several clinical risk scores that had been published for estimating absolute fracture risk over fixed time periods, e.g., 5 or 10 years20-22. The CPG particularly commented on the WHO Fracture Risk Assessment (FRAX®) tool which estimates the 10-year probability of fracture which then can be used to guide decision to treat based on morbidity burden or economic considerations (health care costs and cost effectiveness). At the time of publication, the 2008 CPG opinioned that quantitative data on absolute fracture risk being established by the WHO may not be applicable to all countries as there are international differences in fracture rates and osteoporosis risk factors and that validation studies using such scoring systems on our population would be required.
Pharmacotherapy has been shown to be effective in fracture reduction for patients with either low bone mineral density (T score less than or equal to -2.5 SD) or with fractures. 23-25CPG therefore recommended that all individuals with osteoporosis (T score less than or equal to -2.5 SD) or previous fragility fracture or high absolute risk of fractures should be considered for, and offered appropriate intervention [Grade A, Level 1++, 1+].
The options for treatment include:
i) Oral bisphosphonates
Daily oral bisphosphonates, alendronate and risedronate, have shown to increase BMD and reduce risk of vertebral and hip fractures in postmenopausal osteoporosis23, 24, 26, 27 [Grade A, Level 1++]. Weekly oral regimens of alendronate and risedronate show equivalent increase in BMD as compared to daily dosing, and may be beneficial for improving patient compliance. 28Ibandronate in daily oral dosing has also shown to reduce vertebral fracture risk in postmenopausal osteoporosis,29 and monthly oral regimen may be used which demonstrate better BMD response and patient compliance 30 [Grade A, Level 1++, 1+].
ii) Intravenous bisphosphonates
Ibandronate given as 3 monthly intravenous regimen has shown better BMD response and also may improve patient compliance compared to daily oral regimen. 31 Zoledronic acid given as once yearly intravenous infusion can reduce risk of vertebral, non-vertebral and hip fractures25 [Grade A, Level 1++]. Osteonecrosis of the jaw (ONJ) is a potential complication with intravenous bisphosphonate usage, reported mainly in cancer patients given at high doses, whereas very few cases have been reported with oral bisphosphonates used in the treatment of post-menopausal osteoporosis.
iii) Strontium ranelate
The CPG recommended that strontium ranelate may be used to reduce risk of vertebral fractures and non-vertebral fractures in postmenopausal women 32, 33 [Grade A, Level 1++]. Strontium has also been shown to reduce risk of hip fracture in women aged 74 yrs or older with low BMD (T score less than -3)33. Side effects reported with its usage include gastrointestinal side-effects, increased risk of venous thrombosis and the rare but serious DRESS (Drug Reaction with Eosinophilia and Systemic Symptoms) syndrome.
iv) Raloxifene
Raloxifene is a selective estrogen receptor modulator (SERM) that can reduce vertebral fracture but not non vertebral fracture risk in women [Grade A, Level 1++]. It has the added non-bone advantage of reducing risk of breast cancer in women.34 Concerns of its usage include increased risk of venous thromboembolism.
v) Calcitonin
It prevents bone loss and decreases vertebral fracture risk.35 Data on non-vertebral and hip fracture reduction is based on observational studies.
vi) Teriparatide
Subcutaneous injection of teriparatide (parathyroid hormone 1-34) given daily can enhance bone formation, reduce vertebral and non-vertebral fractures in women with prior vertebral fractures36 [Grade A, Level 1+]. Due to concerns of osteosarcoma in animal studies after lifelong exposure, human use of teriparatide is limited to maximum of 24 months.37
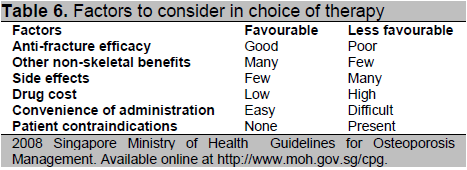
In deciding the most appropriate therapy for osteoporosis, anti-fracture efficacy is a key consideration, on top of which, the following factors should be weighed (Table 6).
Click here to download Table 6
Table 6. Factors to consider in choice of therapy
Under-treatment and poor compliance are key reasons for higher fracture rates, increased morbidity, mortality and cost, and smaller increments in bone
mineral density.38,39 CPG therefore recommended strategies to improve osteoporosis management and adherence to therapy which would include
convenient drug dosing regimens, nurse monitoring, use of educational materials or having educational sessions and referral into a multidisciplinary care
program [Grade C, Level 2+].
Following up on progress after starting treatment is important and the CPG recommended that a useful surrogate marker would be to compare follow-up BMD with baseline BMD, usually at intervals beyond one year 40, 41 [Grade A, Level 1+]. Bone turnover markers were suggested as an alternative method by the CPG to monitor treatment efficacy i.e., to compare baseline levels with that obtained at follow-up at 3 to 6 months after initiation of treatment. This recommendation [Grade A, Level 1+] was based on data that shows that anti-resorptive medications have been associated with reductions from baseline of between 20-40% for bone formation markers such as osteocalcin and bone specific alkaline phosphatase and 30-60% for bone resorption markers such as N-telopeptide, C-telopeptide and deoxypyridinoline.42
In patients who fail to respond, the following should be considered:
i) Non-compliance to therapy
ii) Incorrect administration of drug
iii) On-going, undiagnosed pathology which accelerates bone loss
iv) Imprecision of the BMD measurement technique
v) True treatment failure in which an alternative therapy should be considered
The CPG devoted a separate section to the evaluation and management of male osteoporosis and Glucocorticoid – induced osteoporosis.
i) Male osteoporosis
The CPG recommended that the same diagnostic threshold for osteoporosis in women can be used in men based on evidence that absolute risk for vertebral and hip fractures at any age appears to be similar in men and women of the same age and same BMD43,44 [Grade C, Level 2+]. Secondary causes of osteoporosis are more common in men and hence evaluation for hypogonadism, high alcohol intake, corticosteroid therapy, idiopathic hypercalciuria and other medical disorders associated with secondary osteoporosis should be conducted [Grade C, Level 2+].
Most studies with anti-osteoporosis agents in men are too small to have fracture outcomes.
Instead fracture surrogates like BMD are used with the assumption that if a drug affects the surrogates similarly in men and women, then it is likely that it will also have the same beneficial impact on fracture risk. Options for treatment are thus limited. The CPG in 2008 recommended treating male osteoporosis with alendronate, risedronate or teriparatide 45-47 [Grade A, Level 1+].
ii) Glucocorticoid-induced osteoporosis (GIOP)
Steroids use leads to increased bone resorption and decreased bone formation with increased hip and spine fracture risk within 3 to 6 months after starting glucocorticoid therapy.48,49 Higher dosage of glucocorticoids is associated with greater bone loss and fracture risk. To reduce the risk, dosage and duration of glucocorticoids should be kept to the minimum possible. Lifestyle measures like adequate calcium and vitamin intake and exercise are recommended [GPP]. The CPG recommended that baseline DXA be performed in all individuals who were being initiated on glucorticoid therapy for ≥ 3 months at ≥ 5 mg/day of prednisolone or equivalent and that treatment should be initiated if T-score is less than -1.5 at the femoral neck and /or lumbar spine [Grade C, Level 2+].
Treatment options that were recommended by the CPG for glucocorticoid-induced osteoporosis included alendronate, risedronate and teriparatide all of which have been shown to prevent vertebral fractures in these patients 50-52 [Grade A, Level 1+].
The CPG identified the need to collect and study data on fracture incidence rates as a critical area for research. It also acknowledged the well known fact that hip fracture rates have been the most reliably documented since almost all cases of hip fracture are usually admitted to hospitals. It was opinioned that research into health care costs and effects of diagnostic and therapeutic strategies on fracture reduction would help reveal the cost effectiveness of such strategies. Research into the proportion of individuals with fragility fractures receiving treatment and on the level of osteoporosis awareness and prevalence in the community may reveal the effectiveness of the implementation of case finding strategies.
The CPG proposed that the proportion of patients with prior fragility fracture in adulthood receiving appropriate evaluation for osteoporosis, bone mineral density measurement and appropriate treatment for osteoporosis be considered as pertinent parameters to be considered as part of clinical quality improvement programmes.
At the time of publication of the CPG in 2008, the committee had acknowledged that evidence based CPG’s were only as current as the evidence that supported them and advised that the guidelines be scheduled for review five years after publication or if new evidence appeared that required substantive changes to the recommendations. The following section which does not claim to be exhaustive attempts to highlight some of the clinically relevant updates that have occurred in the field of osteoporosis since the publication of the CPG and that has implications especially in the local context of Singapore.
There is an increasing recognition that the management of osteoporosis and decision to initiate therapy should be based on characterization of absolute fracture risk rather than on bone mineral density alone. FRAX® - the most widely used tool that incorporates clinical risk factors with or without BMD was launched in 2008 and at the time of publication of the CPG, a FRAX® model was not existent in Singapore. However this was rectified in December 2010 when a FRAX® model for Singapore was launched with the unique characteristic of being calibrated to the local epidemiology of fracture and mortality within the 3 main ethnicities of Singapore namely Chinese, Malay and Indian ( http://www.shef.ac.uk/FRAX/tool.jsp?country=35).
FRAX should not be considered as a gold standard in patient assessment but rather as a reference platform that has immense potential for development. Despite its limitations which are several, including lack of a “dose–response” in characteristics like number of prior fractures, the consumption of alcohol, absence of ranges of severity of disease particularly rheumatoid arthritis, non- inclusion of falls risk etc.,53,54 FRAX has the potential to demystify fracture risk assessment in primary care for patients with low bone density, directing clinical fracture prevention strategies to those who can benefit most from them. FRAX has been incorporated into the clinical practice guidelines of some countries and assessment and intervention thresholds have been provided to instruct clinical decision making (e.g.,www.shef.ac.uk/NOGG). These intervention thresholds are likely to be very country specific55-57 and these thresholds will have to be established by individual countries based not only on their fracture rates but also through health economic analyses if feasible. The same holds true in Singapore, where locally validated intervention thresholds based on FRAX estimates have yet to be established.
The CPG in 2008 had recommended that individuals found to have osteoporosis should have relevant clinical, laboratory and radiological assessment to exclude diseases that mimic, cause or aggravate osteoporosis so that appropriate management could be implemented. A list of suggested routine investigations was appended along with the above recommendation. These recommendations were based on studies done in Caucasian populations. The spectrum of diseases and drugs that cause osteoporosis are numerous. However subjecting an individual patient to a vast number of tests may be unnecessary and at the same time costly. It is vital that the commonest secondary causes prevalent in different populations are identified and guidelines be put into place with regard to the most appropriate tests or screening procedures that should be employed to identify these causes. It has recently been shown in Singapore that secondary osteoporosis is more common than previously perceived with an overall prevalence of recognized contributors to secondary osteoporosis like hyperthyroidism, idiopathic hypercalciuria, hypogonadism, vitamin D deficiency etc,, noted to be close to 50% in a population of postmenopausal and older men with osteoporosis 58.
The role of calcium supplementation in osteoporosis management has recently been questioned. Healthy older women randomised to calcium supplementation showed increased rates of myocardial infarction in a randomized controlled trial conducted in 2008.59 This study raised a storm of controversy and was followed by a slew of studies, editorials and commentaries both corroborating with as well as negating the findings. 60-65 However it has to be noted that though a recent meta-analysis of 11 randomized controlled trials 61 demonstrated that the risk of incident myocardial infarction in those patients who were allocated to calcium did increase by 31%, the additional calcium supplementation resulted in an increased risk only in individuals with habitual calcium intake above a median of 805 mg/day and not in those with intakes below this median. This has implications in a country like Singapore where the daily calcium intake of the populace is quite poor averaging only 627 mg.66 Furthermore, though dietary calcium may not confer significant cardiovascular benefit, it does not appear to be associated with an increased risk of cardiovascular events.64 Taken together, all the available data controversial and far from unifying though it may be appear to demonstrate that calcium supplements should be prescribed with caution and only if habitual calcium intake is less than 1000 mg/day. Total (dietary and supplemental) oral calcium intake should not exceed 1000-1500 mg/day.67
Vitamin D deficiency by leading to secondary hyperparathyroidism and increased bone turnover is presumed to be a major contributor to osteoporosis as it leads to secondary hyperparathyroidism and increased bone turnover.68 Controversy in literature has recently erupted following the US Institute of Medicine (IOM) recommendations that the cut-point for serum vitamin D adequacy be 50 nmol/litre rather than the opinion of some experts that it should be 75 nmol/l,69 Practice guidelines subsequently released by the Endocrine Society, agreed with the dietary reference intakes established by the IOM committee and the IOM recommendations not to screen the general population routinely for vitamin D deficiency, However, they also said that serum 25,OH D levels of 30 ng/ml or higher compared with 20 ng/ml would provide increased health benefits; that 30 ng/ml was the desirable level of serum 25, (OH)D level based on the observations that elevated PTH levels were lowered to a plateau when serum 25,OHD was 30 ng/ml or higher; there was a reduction of falls among older persons at serum 25 (OH)D levels of 30 ng/ml or higher and the observation that calcium absorption was maximal at these serum levels of 25(OH)D.70
The relation between vitamin D levels, bone density, and osteoporotic fractures is not clearly defined in all populations71,72 and it may be likely that race-specific ranges of optimal vitamin D are needed. The results of meta-analyses examining the relationship between vitamin D supplementation and fracture reduction also have been inconsistent. Previous meta-analyses have suggested that the benefits of vitamin D may be limited to older persons who live in institutions.73 A recent pooled analyses of 11 double-blind randomized controlled trials showed that vitamin D supplementation of ≥ 800 IU daily was somewhat favourable in the prevention of hip fracture and any nonvertebral fracture in persons 65 years of age or older irrespective of whether they were community dwelling or institutionalized residents. 74 It is likely that earlier trials that showed no benefit with vitamin D supplementation could have been due to lower than intended doses of vitamin D. 75 On the contrary, the trials that showed an unexpected benefit could have been due to higher than intended doses.76 What implications these still conflicting evidence has on practice guidelines in the local context remains to be seen.
Advancement in the molecular pharmacology of osteoporosis is occurring at a rapid rate. The newest bone strengthening drug to win the approval of health regulatory agencies in several countries including that of Singapore is denosumab – a fully humanized monoclonal antibody designed to target the receptor activator of nuclear factor-kappa β ligand (RANKL) a soluble cytokine produced by osteoblasts. RANKL promotes osteoclast differentiation and activation. Denosumab prevents RANKL from engaging the RANK receptor on osteoclasts and osteoclast precursors thereby reducing osteoclast mediated bone resorption and increasing bone density. It is administered by subcutaneous injection (60 mg) every 6 months. Denosumab has been shown to reduce vertebral, hip and non-vertebral fractures.77,78Denosumab’s antiresorptive effects are rapidly reversed once the drug is discontinued and bone density gains seen with its use are also lost. The side effect profile of denosumab includes rashes, eczema and cystitis.77
Osteoporosis is a chronic condition. Robust data regarding the efficacy and safety of both long-term osteoporosis therapy and therapy discontinuation are therefore important. A paucity of clinical trial data regarding the long-term anti-fracture efficacy of osteoporosis therapies necessitates the use of surrogate endpoints in discussions surrounding long-term use and/or discontinuation. Long term safety and efficacy data exist for alendronate [10 years], 79 risedronate [7 years], 80 strontium [8 years],81zoledronic acid [6 years], 82ibandronate [5 years] 83 and denosumab [5 years].84 Data from the trials with bisphosphonates generally suggest that the risk of vertebral fractures is reduced with the continuation of therapy beyond 3-5 years. Consistent evidence of a statistically significant reduction in nonvertebral fractures with the continuation of bisphosphonates is however lacking. With regard to discontinuation of therapy and persistence of benefit, recommendations can be limited to only alendronate 79 and zoledronic acid 82 since clear data from randomized controlled trials only exists for both these agents. Data from these trials show that the bone loss after discontinuation of therapy is only modest as compared with that during continued therapy. For patients who have discontinued treatment after 5 years, there are currently no data to guide clinicians in determining when and whether to resume treatment after the “drug holiday.” The role of repeat assessment of bone mineral density, bone turnover markers and other clinical indicators is currently under study.
Some concerns about long term safety of bisphosphonates have surfaced recently after case series and reports from several countries including Singapore 85 hinted at the occurrence of “atypical femoral fractures” associated with their use. This was subsequently studied and codified into a working diagnosis 86 by the American Society for Bone and Mineral Research (ASBMR). It is still debatable whether bisphosphonates alone are responsible for these atypical fractures which are, compared to typical osteoporosis fractures, considered a rather rare entity and the consensus86,87 is clear that in patients for whom there is justification to treat, the benefits far outweigh the potential rare risks. Physicians are advised to exercise caution and in a patient on bisphosphonate therapy monitor closely for prodromal symptoms like pain in the hip or thigh that may herald the development of atypical fracture.
At the time of publication of the CPG in 2008, data was available only on the use of 3 agents in men-alendronate, risedronate and teriparatide. 45-47 Subsequently, a trial that compared the efficacy and safety of once-yearly intravenous infusion of zoledronic acid versus once weekly oral alendronate showed that both bisphosphonates increased spine bone density by over 6% at 2 years. 88 In a post-hip fracture trial, zoledronic acid compared with placebo infusion lowered the subsequent clinical fracture rate in men and women.89A very recent multicentre randomized trial has now shown that zoledronic acid treatment is associated with a significantly reduced risk of vertebral fracture among men with osteoporosis .90 Recently the EMA (European Medicines Agency) approved the use of strontium ranelate for treating male osteoporosis based on a bridging study which showed that daily strontium increased bone density more than weekly alendronate. 91 Denosumab is also approved in Europe for men on androgen deprivation therapy for prostate cancer based on a large randomized controlled trial of almost 1500 men which showed that those who received denosumab had significant increases in BMD in the spine, hip and interestingly the distal one third radius. In addition, those men who received denosumab had fewer morphometric vertebral fractures. 92 The impact of the increase in radius BMD is not known but this is the first novel agent that appears to do so. A recent small study investigating the effects of denosumab on 242 men with low BMD showed that one year of denosumab therapy caused a reduction in bone resorption and significant increases in BMD at all skeletal sites assessed.93
Breaking the “fragility fracture cycle” is a major challenge. The beneficial impact of centralized secondary fracture prevention programs on post fracture osteoporosis management is being recognized. Interventions based on public and health education alone are unlikely to improve osteoporosis management. Coordinator based systems circumvent the challenge of where clinical responsibility resides for care of the fragility fracture patient. 94,95 A Ministry of Health (MOH) funded osteoporosis disease management program – OPTIMAL (Osteoporosis Patient Targeted and Integrated Management for Active Living) was launched in Singapore in 2008. This programme targets high-risk patients who previously have had fragility fractures and was implemented employing the principles of chronic disease management i.e., risk stratification, evidence based guidelines, case management and outcomes tracking. Patients enrolled into the OPTIMAL program benefit from among other things, screening with DXA, osteoporosis education and close follow up over the first 2 years following recruitment. Falls prevention strategies constitute an important element of OPTIMAL and a structured program - the OTAGO exercise program that provides 10 one-hour sessions of balance and strengthening exercises training with recommendations for continuing at home/community gym or individual PT over the next 2 years is offered to all patients enrolled into the program if they are deemed suitable for participation The OPTIMAL program has succeeded in identifying, evaluating and treating a large number of patients with fragility fractures in Singapore to date.96 The ultimate success of the program will have to be measured by fractures prevented over long term follow up and cost effectiveness.
Health care in Singapore is provided through both public and private services. No published data exists on the rates of utilization of the CPG in the community. There is a pressing need to identify the unmet needs in osteoporosis care and barriers if present to osteoporosis identification and treatment and to find out whether focus should be shifting from education and preventive measures amongst the public to support for physicians through provision of more resources and modification of existing systems of care. DXA is widely available in Singapore. At the time of the publication of the CPG in 2008, it was estimated that 14 DXA machines were available for use in the island country. Currently there are 27 DXA machines available to service a population of approximately 5 million in Singapore. However studies have not been done on whether this easily available service is made use of and whether guidelines on the proper usage and interpretation of DXA are being followed. A survey conducted in 2010 amongst health care professionals from across the Asia-Pacific region97 which included physicians from within Singapore showed up the rather concerning finding that several aspects of what can be considered as up to date osteoporosis care including screening for secondary causes, employing risk calculating tools etc., are still not being given due importance despite these being emphasized in the CPG. As such there is quite an urgent need to evaluate the effectiveness of the Clinical Practice Guidelines and to increase engagement of both primary care as well as specialist physicians in the adoption of the guidelines. Cost- effective tools such as postal surveys with incentives for participation can be considered to monitor CPG usage and obtain feedback on barriers to compliance with the recommendations. 98
No large scale health economic study has been conducted in Singapore. However a comparatively small study in 200899 estimated that the mean hospitalisation cost of hip fracture was approximately S$10,515, which highlights the direct economic burden of the disease. Singapore’s health care financing system is anchored on the twin philosophies of individual responsibility and affordable health care to all. Subsidies based on the financial class of the patient and funded by the Government exist for inpatient care and outpatient follow-up. However, osteoporosis treatment, including medication costs, are currently by and large borne by the individual patient. Medications used in the treatment of osteoporosis remain comparatively expensive and this factor may contribute to non compliance by patients which may then potentially increase the risk of fractures98 and thus indirectly contribute to health care costs. However it is heartening to note that a study conducted in Singapore in 2012 estimating the compliance and persistence to prescribed bisphosphonate therapy amongst patients at the largest public restructured hospital in Singapore showed higher adherence rates to therapy in comparison to studies conducted in the US and Europe.100-103 Whether this is applicable for other anti-osteoporosis medications and whether these findings hold true in the community setting remains to be studied. More data also would be warranted to assess the actual health care burden imposed by osteoporosis and it’s complications in Singapore. The cost effectiveness of the Secondary Fracture Prevention programme instituted in 2008 also deserves immediate study.
Clinical guidelines should provide recommendations that are lucidly formulated, reliable, clinically applicable and acceptable to the end users i.e., the health care professionals who have to implement them in daily practice. High-quality guidelines for the management of osteoporosis are available in Singapore. The CPG published in 2008 emphasized the importance of evidence based medicine and the value of an integrative approach to diagnosing and treating osteoporosis. The challenge now lies in incorporating the numerous advances in the field of osteoporosis as well as data unique to our population into subsequent revisions as well as to increase the acceptability of CPG’s amongst health care professionals.
1. Committee on Ageing Issues: Report on Aging Population. 2006.
2. Lau EM. Epidemiology of osteoporosis. Best Pract Res Clin Rheumatol 2001;15(3):335-344.
3. Koh LK, Saw SM, Lee JJ, Leong KH, Lee J, National Working Committee on Osteoporosis, et al. Hip fracture incidence rates in Singapore 1991-1998. Osteoporos Int 2001;12(4):311-318.
4. Thoo FL, Chng SM, Lam KS, Lee JBI, Tan MC, Teh HS, et al. To establish the normal bone mineral density reference database for the Singapore male. Ann Acad Med Singapore 2002;31(1):21-25.
5. Leong KH, Feng PH. Bone mineral density measurements using the Hologic QD2000 in 175 Singaporean women aged 20-80. Singapore Med J 1997;38(1):25-26.
6. Koh LK, Sedrine WB, Torralba TP, Kung A, Fujiwara S, Chan SP, et al. A simple tool to identify Asian women at increased risk of osteoporosis. Osteoporos Int 2001;12(8):699-705.
7. Ho SC, Leung PC, Swaminathan R, Chan C, Chan SS, Fan YK, et al. Determinants of bone mass in Chinese women aged 21-40 years. II. Pattern of dietary calcium intake and association with bone mineral density. Osteoporos Int 1994;4(3):167-175.
8. Chan HH, Lau EM, Woo J, Lin F, Sham A, Leung PC, et al. Dietary calcium intake physical activity and the risk of vertebral fracture in Chinese. Osteoporos Int 1996;6(3):228-232.
9. Cumming RG, Nevitt MC. Calcium for prevention of osteoporotic fractures in postmenopausal women. J Bone Miner Res 1997;12(9):1321-1329.
10. Bischoff-Ferrari HA, Willett WC, Wong JB, Giovannucci ED, Dietrich T, -Hughes BD, et al. Fracture prevention with vitamin D supplementation a meta-analysis of randomized controlle d trials. JAMA 2005;293(18):2257-2264.
11. Bischoff-Ferrari HA, -Hughes BD, Willett WC, Staehelin HB, Bazemore MG, Zee RY, et al. Effect of Vitamin D on falls a meta-analysis. JAMA 2004;291(16):1999-2006.
12. Shea B, Bonaiuti D, Iovine R, Negrini S, Robinson V, Kemper HC, et al. Cochrane Review on exercise for preventing and treating osteoporosis in postmenopausal women. Eura Medicophys 2004;40(3):199-209.
13. Englund U, Littbrand H, Sondell A, Pettersson U, Bucht G. A 1-year combined weight-bearing training program is beneficial for bone mineral density and neuromuscular function in older women. Osteoporos Int 2005;16(9):1117-1123.
14. Daniell HW. Osteoporosis of the slender smoker. Vertebral compression fractures and loss of metacarpal cortex in relation to postmenopausal cigarette smoking and lack of obesity. Arch Intern Med 1976;136(3):298-304.
15. Hernandez-Avila M, Colditz GA, Stampfer MJ, Rosner B, Speizer FE, Willett WC, et al. Caffeine moderate alcohol intake and risk of fractures of the hip and forearm in middle-aged women. Am J Clin Nutr 1991;54(1):157-163.
16. Parker MJ, Gillespie WJ, Gillespie LD. Effectiveness of hip protectors for preventing hip fractures in elderly people systematic review. BMJ 2006;332(7541):571-574.
17. Sawka AM, Boulos P, Beattie K, Thabane L, Papaioannou A, Gafni A, et al. Do hip protectors decrease the risk of hip fracture in institutional and community-dwelling elderly? A systematic review and meta-analysis of randomized controlled trials. Osteoporos Int 2005;16(12):1461-1474.
18. Gillespie LD, Gillespie WJ, Robertson MC, Lamb SE, Cumming RG, Rowe BH, et al. Interventions for preventing falls in elderly people. Cochrane Database Syst Rev 2003;(4):CD000340.
19. Chang JT, Morton SC, Rubenstein LZ, Mojica WA, Maglione M, Suttorp MJ, et al. Interventions for the prevention of falls in older adults. Systematic review and meta-analysis of randomised clinical trials. BMJ Clinical research ed.) 2004;328(7441):680.
20. Nguyen ND, Frost SA, Center JR, Eisman JA, Nguyen TV. Development of a nomogram for individualizing hip fracture risk in men and women. Osteoporos Int 2007;18(8):1109-1117.
21. Kanis JA, Borgstrom F, De Laet C, Johansson H, Johnell O, Jonsson B, et al. Assessment of fracture risk. Osteoporos Int 2005;16(6):581-589.
22. Kung AWC, Lee KK, Ho AY, Tang G, Luk KD. Ten-year risk of osteoporotic fractures in postmenopausal Chinese women according to clinical risk factors and BMD T-scores: A prospective study. J Bone Miner Res 2007;22(7):1080-1087.
23. Black DM, Cummings SR, Karpf DB, Cauley JA, Thompson DE, Nevitt MC, et al. Randomised trial of effect of alendronate on risk of fracture in women with existing vertebralfractures. Fracture Intervention Trial Research Group. Lancet 1996;348(9041):1535-1541.
24. McClung MR, Geusens P, Miller PD, Zippel H, Bensen WG, Roux C, et al. Effect of risedronate on the risk of hip fracture in elderly women. Hip Intervention Program Study Group. N Engl J Med. 2001 Feb 1;344(5):333-40.
25. Black DM, Delmas PD, Eastell R, Reid IR, Boonen S, Cauley JA, et al. Once-yearly zoledronic acid for treatment of postmenopausal osteoporosis. N Engl J Med 2007;356(18):1809-1822.
26. Harris ST, Watts NB, Genant HK, McKeever CD, Hangartner T, Keller M, et al. Effects of risedronate treatment on vertebral and nonvertebral fractures in women with postmenopausal osteoporosis: A randomized controlled trial. Vertebral Efficacy With Risedronate Therapy (VERT) Study Group. JAMA.1999 Oct 13;282(14):1344-52.
27. Black DM, Thompson DE, Bauer DC, Ensrud K, Musliner T, Hochberg MC, et al. Fracture risk reduction with alendronate in women with osteoporosis: The Fracture Intervention Trial. FIT Research Group. J Clin Endocrinol Metab. 2000 Nov;85(11):4118-24.
28. Cramer JA, Gold DT, Silverman SL, Lewiecki EM. A systematic review of persistence and compliance with bisphosphonates for osteoporosis. Osteoporos Int 2007;18(8):1023-1031.
29. Chesnut IC, Skag A, Christiansen C, Recker R, Stakkestad JA, Hoiseth A, et al. Effects of oral ibandronate administered daily or intermittently on fracture risk in postmenopausal osteoporosis. J Bone Miner Res. 2004 Aug;19(8):1241-9.
30. Reginster JY, Adami S, Lakatos P, Greenwald M, Stepan JJ, Silverman SL, et al. Efficacy and tolerability of once-monthly oral ibandronate in postmenopausal osteoporosis 2 year results from the MOBILE study. Ann Rheum Dis 2006;65(5):654-661.
31. Adami S, Felsenberg D, Christiansen C, Robinson J, Lorenc RS, Mahoney P, et al. Efficacy and safety of ibandronate given by intravenous injection once every 3 months. Bone 2004;34(5):881-889.
32. Meunier PJ, Roux C, Seeman E, Ortolani S, Badurski JE, Spector TD, et al. The effects of strontium ranelate on the risk of vertebral fracture in women with postmenopausal osteoporosis. N Engl J Med 2004;350(5):459-468.
33. Reginster JY, Seeman E, Vernejoul MCD, Adami S, Compston J, Phenekos C, et al. Strontium ranelate reduces the risk of non vertebral fractures in postmenopausal women with osteoporosis Treatment of Peripheral Osteoporosis (TROPOS) study. J Clin Endocrinol Metab 2005;90(5):2816-2822.
34. Ettinger B, Black DM, Mitlak BH, Knickerbocker RK, Nickelsen T, Genant HK, et al. Reduction of vertebral fracture risk in postmenopausal women with osteoporosis treated with raloxifene results from a 3-year randomized clinical trial. Multiple Outcomes of Raloxifene Evaluation (MORE) Investigators. JAMA 1999;282(7):637-645.
35. Chesnut CH, Silverman S, Andriano K, Genant H, Gimona A, Harris S, et al. A randomized trial of nasal spray salmon calcitonin in postmenopausal women with established osteoporosis the prevent recurrence of osteoporotic fractures study. PROOF Study Group. Am J Med 2000;109(4):267-276.
36. Neer RM, Arnaud CD, Zanchetta JR, Prince R, Gaich GA, Reginster JY, et al. Effect of parathyroid hormone (1-34) on fractures and bone mineral density in postmenopausal women with osteoporosis. N Engl J Med 2001;344(19):1434-1441.
37. Vahle JL, Long GG, Sandusky G, Westmore M, Ma YL, Sato M, et al. Bone neoplasms in F344 rats given teriparatide [rhPTH(1-34)] are dependent on duration of treatment and dose. Toxicol Pathol 32(4):426-438.
38. Elliot-Gibson V, Bogoch ER, Jamal SA, Beaton DE. Practice patterns in the diagnosis and treatment of osteoporosis after a fragility fracture: A systematic review. Osteoporos Int. 2004;15(10):767-778.
39. Seeman E, Compston J, Adachi J, Brandi ML, Cooper C, Dawson-Hughes B, et al. Non-compliance the Achilles heel of anti-fracture efficacy. Osteoporos Int. 2007;18(6):711-719.
40. Leib ES, Lenchik L, Bilezikian JP, Maricic MLJ, Watts NLB. Position statements of the International Society for Clinical Densitometry: methodology. J Clin Densitom 2002;5 Suppl:S5-10.
41. Wasnich RD, Miller PD. Antifracture efficacy of antiresorptive agents are related to changes in bone density. J Clin Endocrinol Metab 2000;85(1):231-236.
42. Delmas PD, Eastell R, Garnero P, Seibel MJ, Stepan J. The use of biochemical markers of bone turnover in osteoporosis. Committee of Scientific Advisors of the International Osteoporosis Foundation. Osteoporosis Int 2000;11 Suppl 6:S2-17.
43. Seeman E, Bianchi G, Khosla S, Kanis JA, Orwoll E. Bone fragility in men-where are we? Osteoporos Int. 2006;17(11):1577-83.
44. Kanis JA, Johnell O, Oden A, Dawson A, De Laet C, Jonsson B. Ten year probabilities of osteoporotic fractures according to BMD and diagnostic thresholds. Osteoporos Int. 2001 Dec;12(12):989-95.
45. Ringe JD, Dorst A, Faber H, Ibach K. Alendronate treatment of established primary osteoporosis in men: 3-year results of a prospective, comparative, two-arm study. Rheumatol Int. 2004 Mar;24(2):110-3.
46. Ringe JD, Faber H, Farahmand P, Dorst A. Efficacy of risedronate in men with primary and secondary osteoporosis: Results of a 1-year study. Rheumatol Int. 2006 Mar;26(5):427-31.
47. Kaufman JM, Orwoll E, Goemaere S, San Martin J, Hossain A, Dalsky GP, et al. Teriparatide effects on vertebral fractures and bone mineral density in men with osteoporosis: Treatment and discontinuation of therapy. Osteoporos Int. 2005 May;16(5):510-6.
48. van Staa TP, Leufkens HG, Cooper C. The epidemiology of corticosteroid- induced osteoporosis: A meta-analysis. Osteoporos Int. 2002 Oct;13(10):777-87.
49. van Staa TP, Leufkens HG, Abenhaim L, Zhang B, Cooper C. Use of oral corticosteroids and risk of fractures. J Bone Miner Res. 2000 Jun;15(6):993-1000.
50. Adachi JD, Saag KG, Delmas PD, Liberman UA, Emkey RD, Seeman E, et al. Two-year effects of alendronate on bone mineral density and vertebral fracture in patients receiving glucocorticoids: A randomized, double-blind, placebo-controlled extension trial. Arthritis Rheum 2001;44(1):202-211.
51. Cohen S, Levy RM, Keller M, Boling E, Emkey RD, Greenwald M, et al. Risedronate therapy prevents corticosteroid-induced bone loss. A twelve-month, multicenter, randomized, double-blind, placebo-controlled, parallel-group study. Arthritis and Rheumatism 1999;42(11):2309-2318.
52. Lane NE, Sanchez S, Modin GW, Genant HK, Pierini E, Arnaud CD. Parathyroid hormone treatment can reverse corticosteroid-induced osteoporosis. Results of a randomized controlled clinical trial. J Clin Invest.1998 Oct 15;102(8):1627-33.
53. Cauley JA, El-Hajj Fuleihan G, Arabi A, Fujiwara S, Ragi-Eis S, Calderon A, et al. Official Positions for FRAX® clinical regarding international differences from Joint Official Positions Development Conference of the International Society for Clinical Densitometry and International Osteoporosis Foundation on FRAX®. J Clin Densitom 2011;14(3):240-262.
54. Hans DB, Kanis JA, Baim S, Bilezikian JP, Binkley N, Cauley JA, et al. Joint Official Positions of the International Society for Clinical Densitometry and International Osteoporosis Foundation on FRAX(®). Executive Summary of the 2010 Position Development Conference on Interpretation and use of FRAX® in clinical practice. J Clin Densitom 2011;14(3):171-180.
55. Lippuner K, Johansson H, Borgström F, Kanis JA, Rizzoli R. Cost-effective intervention thresholds against osteoporotic fractures based on FRAX® in Switzerland. Osteoporos Int 2012; Jan 6 (Epub ahead of print)
56. Johansson H, Kanis JA, McCloskey EV, Odén A, Devogelaer JP, Kaufman JM, et al. A FRAX® model for the assessment of fracture probability in Belgium. Osteoporos Int 2011;22(2):453-461.
57. Kanis JA, Johansson H, Odén A, McCloskey EV. The distribution of FRAX(®)-based probabilities in women from Japan. J Bone Miner Metab 2012; Aug 22 (Epub ahead of print).
58. Chee Kwang Yung, Stephanie Fook-Chong, Manju Chandran. The prevalence of recognized contributors to secondary osteoporosis in South East Asian men and post-menopausal women. Are Z score diagnostic thresholds useful predictors of their presence? Arch Osteoporos 2012 DOI10.1007/s11657-012-0078-z.
59. Bolland MJ, Barber PA, Doughty RN, Mason B, Horne A, Ames R, et al. Vascular events in healthy older women receiving calcium supplementation: Randomised controlled trial. BMJ 2008;336(7638):262-266.
60. Bolland MJ, Grey A, Avenell A, Gamble GD, Reid IR. Calcium supplements with or without vitamin D and risk of cardiovascular events: Reanalysis of the Women's Health Initiative limited access dataset and meta-analysis. BMJ 2011;342:d2040.
61. Bolland MJ, Avenell A, Baron JA, Grey A, MacLennan GS, Gamble GD, et al. Effect of calcium supplements on risk of myocardial infarction and cardiovascular events: Meta-analysis. BMJ 2010;341:c3691.
62. Abrahamsen B, Sahota O. Do calcium plus vitamin D supplements increase cardiovascular risk? BMJ 2011;342:d2080.
63. Lewis JR, Calver J, Zhu K, Flicker L, Prince RL. Calcium supplementation and the risks of atherosclerotic vascular disease in older women: Results of a 5-year RCT and a 4.5-year follow-up. J Bone Miner Res 2011;26(1):35-41.
64. Li K, Kaaks R, Linseisen J, Rohrmann S. Associations of dietary calcium intake and calcium supplementation with myocardial infarction and stroke risk and overall cardiovascular mortality in the Heidelberg cohort of the European Prospective Investigation into Cancer and Nutrition study (EPIC-Heidelberg). Heart 2012;98(12):920-925.
65. Nordin BEC, Lewis JR, Daly RM, Horowitz J, Metcalfe A, Lange K, et al. The calcium scare-what would Austin Bradford Hill have thought? Osteoporos Int 2011;22(12):3073-3077.
66. Lee YHD, Lim YW, Ling PS, Tan YY, Cheong M, Lam KS, et al. Inadequate dietary calcium intake in elderly patients with hip fractures. Singapore Med J 2007;48(12):1117-1121.
67. Zittermann A, Pilz S, Börgermann J, Gummert JF. Calcium supplementation and vitamin D: A trigger for adverse cardiovascular events? Future cardiology 2011;7(6):725-727.
68. Lips P, Duong T, Oleksik A, Black D, Cummings S, Cox D, et al. A global study of vitamin D status and parathyroid function in postmenopausal women with osteoporosis: Baseline data from the multiple outcomes of raloxifene evaluation clinical trial. J Clin Endocrinol Metab 2001;86(3):1212-1221.
69. Ross AC, Manson JE, Abrams SA, Aloia JF, Brannon PM, Clinton SK, et al. The 2011 report on dietary reference intakes for calcium and vitamin D from the Institute of Medicine: What clinicians need to know. J Clin Endocrinol Metab 2011;96(1):53-58.
70. Holick MF, Binkley NC, Bischoff-Ferrari HA, Gordon CM, Hanley DA, Heaney RP, et al. Evaluation, treatment, and prevention of vitamin D deficiency: An Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 2011;96(7):1911-1930.
71. Gutiérrez OM, Farwell WR, Kermah D, Taylor EN. Racial differences in the relationship between vitamin D, bone mineral density, and parathyroid hormone in the National Health and Nutrition Examination Survey. Osteoporos Int 2011;22(6):1745-1753.
72. Chandran M, Hoeck HC, Wong HC, Zhang RF, Dimai HP. Vitamin D status and its relationship with bone mineral density and parathyroid hormone in Southeast Asian adults with low bone density. Endocr Pract 2011;17(2):226-234.
73. Bischoff-Ferrari HA, Willett WC, Wong JB, Giovannucci ED, Dietrich T, -Hughes BD, et al. Fracture prevention with vitamin D supplementation, a meta-analysis of randomized controlled trials. JAMA 2005;293(18):2257-2264.
74. Bischoff-Ferrari HA, Willett WC, Orav EJ, Oray EJ, Lips P, Meunier PJ, et al. A pooled analysis of vitamin D dose requirements for fracture prevention. N Engl J Med 2012;367(1):40-49.
75. Grant AM, Avenell A, Campbell MK, McDonald AM, MacLennan GS, McPherson GC, et al. Oral vitamin D3 and calcium for secondary prevention of low-trauma fractures in elderly people (Randomised Evaluation of Calcium Or vitamin D, RECORD): a randomised placebo-controlled trial. Lancet 2005;365(9471):1621-1628.
76. Jackson RD, LaCroix AZ, Gass M, Wallace RB, Robbins J, Lewis CE, et al. Calcium plus vitamin D supplementation and the risk of fractures. N Engl J Med 2006;354(7):669-683.
77. Cummings SR, San Martin J, McClung MR, Siris ES, Eastell R, Reid IR, et al. Denosumab for prevention of fractures in postmenopausal women with osteoporosis. N Engl J Med 2009;361(8):756-765.
78. Reid IR, Miller PD, Brown JP, Kendler DL, Fahrleitner-Pammer A, Valter I, et al. Effects of denosumab on bone histomorphometry: The FREEDOM and STAND studies. J Bone Miner Res 2010;25(10):2256-2265.
79. Black DM, Schwartz AV, Ensrud KE, Cauley JA, Levis S, Quandt SA, et al. Effects of continuing or stopping alendronate after 5 years of treatment: The Fracture Intervention Trial Long-term Extension (FLEX): a randomized trial. JAMA 2006;296(24):2927-2938.
80. Mellström DD, Sörensen OH, Goemaere S, Roux C, Johnson TD, Chines AA, et al. Seven years of treatment with risedronate in women with postmenopausal osteoporosis. Calcif Tissue Int 2004;75(6):462-468.
81. Reginster JY, Bruyère O, Sawicki A, Roces-Varela A, Fardellone P, Roberts A, et al. Long-term treatment of postmenopausal osteoporosis with strontium ranelate: Results at 8 years. Bone 2009;45(6):1059-1064.
82. Black DM, Reid IR, Boonen S, Bucci-Rechtweg C, Cauley JA, Cosman F, et al. The effect of 3 versus 6 years of zoledronic acid treatment of osteoporosis: A randomized extension to the HORIZON-Pivotal Fracture Trial (PFT). J Bone Miner Res 2012;27(2):243-254.
83. Miller PD, Recker RR, Reginster JY, Riis BJ, Czerwinski E, Masanauskaite D, et al. Efficacy of monthly oral ibandronate is sustained over 5 years: The MOBILE long-term extension study. Osteoporos Int 2012;23(6):1747-1756.
84. Papapoulos S, Chapurlat R, Libanati C, Brandi ML, Brown JP, Czerwiński E, et al. Five years of denosumab exposure in women with postmenopausal osteoporosis: Results from the first two years of the FREEDOM extension. J Bone Miner Res 2012;27(3):694-701.
85. Goh SK, Yang KY, Koh JSB, Wong MK, Chua SY, Chua DTC, et al. Subtrochanteric insufficiency fractures in patients on alendronate therapy: A caution. J Bone Joint Surg Br 2007;89(3):349-353.
86. Shane E, Burr D, Ebeling PR, Abrahamsen B, Adler RA, Brown TD, et al. Atypical subtrochanteric and diaphyseal femoral fractures report of a task force of the American Society for Bone and Mineral Research. J Bone Miner Res 2010;25(11):2267-2294.
87. Rizzoli R, Akesson K, Bouxsein M, Kanis JA, Napoli N, Papapoulos S, et al. Subtrochanteric fractures after long-term treatment with bisphosphonates: A European Society on Clinical and Economic Aspects of Osteoporosis and Osteoarthritis, and International Osteoporosis Foundation Working Group Report. Osteoporos Int 2011;22(2):373-390.
88. Orwoll ES, Miller PD, Adachi JD, Brown J, Adler RA et al. Efficacy and safety of once-yearly i.v. infusion of zoledronic acid 5 mg versus once-weekly 70 mg oral alendronate in the treatment of male osteoporosis: A randomized, multicenter, double blind, active-controlled study. J Bone Miner Res 25:2239-2250.
89. Lyles KW, Colon-Emeric CS, Magaziner JS, Adachi JD, Pieper CF, Mautalen C et al. Zoledronic acid in reducing clinical fracture and mortality after hip fracture. N Engl J Med 2007; 357:nihpa40967. DOI:10.1056/NEJMe074941.
90. Boonen S, Reginster JY, Kaufman JM, Lippuner K, Zanchetta J et al. Fracture risk and zoledronic acid therapy in men with osteoporosis. N Engl J Med 2012; Nov; 367(18):1714-23.
91. Ringe JD, Dorst A and Farahmand P. Efficacy of strontium ranelate on bone mineral density in men with osteoporosis. Arzneimittelforschung 2010; 60:267-272.
92. Smith MR, Egerdie B and Toriz NH. Denosumab in men receiving androgen-deprivation therapy for prostate cancer. N Engl J Med 361:756-765.
93. Orwoll E, Teglbjaerg CS, Langdahl BL, Chapurlat R, Czerwinski E et al. A randomized, placebo-controlled study of the effects of denosumab for the treatment of men with low bone mineral density. J Clin Endocrinol Metab 2012 Sep;97(9):3161-9.
94. Marsh D, Akesson K, Beaton DE, Bogoch ER, Boonen S, Brandi ML, et al. Coordinator-based systems for secondary prevention in fragility fracture patients. Osteoporos Int 2011;22(7):2051-2065.
95. Adler RA. Secondary fracture prevention. Curr Osteoporos Rep 2012;10(1):22-27.
96. Manju Chandran and Matthew Tan. Addressing the care gap in secondary fracture prevention in a South East Asian hospital-Effective initiation of osteoporosis diagnosis and treatment for patients with fragility fractures through “OPTIMAL”. Osteoporos Int 2012; 23(Suppl2) DOI10.1007/S00198-012-1923-7.
97. Daniela Korthoewer and Manju Chandran (On behalf of the Endocrine and Metabolic Society of Singapore). Osteoporosis management and the utilization of FRAX(R): Asurvey amongst health care professionals of the Asia-Pacific. Arch Osteoporosis 2012; DOI 10.1007/s11657-012-0097-9.
98. Hutchinson A, McIntosh A, Cox S, Gilbert C. Towards efficient guidelines. How to monitor guideline use in primary care. Health Technol Assess 2003; 7(iii):1-97.
99. Lee YH, Lim YW, Lam KS. Economic cost of osteoporotic hip fractures in Singapore. Singapore Medical Journal 2008;49(12):980-984.
100. Siris ES, Harris ST, Rosen CJ, Barr CE, Arvesen JN, Abbott TA, Silverman S. Adherence to bisphosphonate therapy and fracture rates in osteoporotic women: Relationship to vertebral and non vertebral fractures from 2 US claims databases. Mayo Clin Proc 2006; 81(8):1013-1022.
101. M.H.H Cheen, M.C. Kong, R.F Zhang, F.M.H Tee, M. Chandran. Adherence to osteoporosis medications amongst Singaporean patients. Osteoporos Int 2012; 23:1053-1060.
102. Solomon DH, Avorn J, Katz JN, Finkelstein JS, Arnold M, Polinski JM, Brookhart MA. Compliance with osteoporosis medications. Arch Intern Med 2005; 165(20):2414-2419.
103. Bocuzzi S, Foltz S, Omar M. Assessment of adherence and persistence with daily and weekly dosing regimens of oral bisphosphonates. Osteoporos Int 2005; 16:S35-S36.