Overweight and obesity is defined by the World Health Organization (WHO) as a Body Mass Index (BMI) equal to or more than 25 kg/m2, and BMI equal to or more than 30 kg/m2, respectively. Both are associated with several health-related problems such as hypertension, dyslipidemia, type 2 diabetes mellitus and cardiovascular disease, among others. The WHO projects that by 2015, approximately 2.3 billion adults will be overweight and more than 700 million will be obese. Overweight and obesity lead to serious health consequences and the risk increases progressively as BMI increases. The relationship of obesity with type 2 diabetes has been recognized for decades, and the major basis for this association is the ability of obesity to produce insulin resistance.1 The risk for diabetes and presumably insulin resistance, as shown by large epidemiologic studies, ri ses as body fat content (measured by BMI) increases from the very lean to the very obese.2 This implies that the amount of body fat has an effect on insulin sensitivity across a broad range. In obese individuals, adipose tissue releases increased amounts of non-esterified fatty acids, glycerol, hormones, pro-inflammatory cytokines and other factors that are involved in the development of insulin resistance.
Several lines of evidence indicate that insulin resistance compromises endothelial function. Decreased bioavailability of nitric oxide (NO) is a major factor for the development of endothelial dysfunction and plays an important role in the development of insulin resistance. The activity of endothelium-derived NO synthase (NOS), which regulates blood flow to insulin-sensitive tissues, is impaired in insulin-resistant individuals. Inhibition of NOS impairs microvascular recruitment and blunts muscle glucose uptake in response to insulin. Insulin resistance as a marker for peripheral endothelial dysfunction can be explained by several mechanisms. The endothelium has important roles both in the delivery of insulin to the tissues and as a target for insulin action. Defects in insulin stimulation of muscle perfusion and insulin signalling in the skeletal muscle cell contribute to metabolic insulin resistance under conditions of decreased NO bioavailability.3 Insulin is an arterial vasodilator in skeletal muscle vascular beds. There is evidence that insulin-mediated vasodilation is reduced in states of insulin resistance. An impaired insulin-mediated increase in skeletal muscle blood flow has been described in insulin-resistant states, including obesity, hypertension and type 2 diabetes mellitus. Reduced capillary surface area and impaired capillary endothelial function, along with a failure of endothelial vasodilator response to insulin in arterioles, could contribute to insulin resistance through delayed insulin delivery to the interstitial fluid.4
Given the impact of eating habits on cardiovascular disease, significant emphasis has recently been placed on identifying foods that have beneficial effects on health. The flavanols, a class of plant-derived polyphenols, have been proposed as likely candidates, given the link between an increased dietary intake of these phytochemicals and a reduction in cardiovascular events.5 The flavanols comprise several distinct subclasses which are present in different concentrations in various foods, including, but not limited to: red wine, black tea, onions, apples and cocoa. Cocoa and chocolate contain both a high quantity and quality of antioxidant flavanols, even exceeding black and green tea and red wine. The amount of flavanols in chocolate is not only dependent on the cacao bean itself, but also on the processing steps involved in its manufacture. Dark chocolate contains 2 to 3 times as many cocoa flavanols as milk chocolate. Plasma flavanol concentrations become detectable with peak concentrations at 60 minutes after intake and have a half-life of 3.6 hours.
A 2008 meta-analysis of randomized controlled trials by Hooper et al on flavonoids, flavonoid-rich foods and cardiovascular risk showed that chocolate and cocoa increased flow-mediated dilatation (FMD), a measure of endothelial function, and reduced systolic and diastolic blood pressures after acute and chronic intake.6 Desch and colleagues, in a meta-analysis from 2010, confirmed the antihypertensive effect of cocoa products. 7 The proposed mechanism was an increase in the bioavailability of vasodilating nitric oxide, possibly caused, in part, by an enhanced nitric oxide synthase activity. Grassi and colleagues in 2005 showed that polyphenol-rich dark chocolate, but not white chocolate, decreases blood pressure and improves insulin sensitivity in healthy persons. They concluded that these findings indicate that dark chocolate may exert a protective action on the vascular endothelium by improving insulin sensitivity.8 Because a decrease in NO bioavailability leads to the development of endothelial dysfunction—a major factor for the development of insulin resistance—flavanol-rich cocoa, shown to increase NO bioavailability, would therefore increase insulin sensitivity or decrease insulin resistance. This is consistent with studies that showed polyphenol-rich dark chocolate, regardless of the dose, reduced fasting blood glucose levels and blood pressure in overweight an obese individuals. 9 These support previous observations that polyphenol-rich dark chocolate intake improved insulin resistance, insulin sensitivity, fasting glucose levels and blood pressure in healthy individuals, hypertensives, glucose-intolerant hypertensives and obese subjects. Other studies have also shown that the acute ingestion of either solid dark chocolate or liquid cocoa significantly improves endothelial function and lowers blood pressure in healthy, overweight adults.10
There have been several studies on cocoa consumption and insulin sensitivity/resistance involving overweight and obese subjects. These studies had small sample sizes and conflicting results. This review aims to assess the effects of flavanol-rich cocoa for insulin sensitivity in overweight and obese individuals.
Both QUICKI and HOMA are well-validated indices of insulin sensitivity.11,12 The glucose clamp technique is widely accepted as the reference standard for directly determining metabolic insulin sensitivity in humans.13 This is done by using a bedside glucose analyzer to monitor blood glucose levels during a constant insulin infusion after an overnight fast while 20% dextrose is given intravenously at a variable rate to “clamp” blood glucose concentrations in the normal range. Its application in larger studies may be limited because it is time consuming, labor intensive, expensive and requires an experienced operator to manage technical difficulties. Thus, for epidemiological studies, large clinical investigations, or routine clinical application, the glucose clamp is not appropriate.14 There is a good correlation of QUICKI with the glucose clamp technique. It is an empirically derived mathematical transformation of fasting blood glucose and fasting plasma insulin concentrations:
QUICKI = 1 / [log (fasting insulin) + log (fasting glucose)]
Fasting insulin is expressed in U/mL, and fasting glucose in mg/dL. Excellent linear correlations between QUICKI and glucose clamp estimates in healthy subjects, obesity, diabetes, hypertension, and many other insulin-resistant states are found in several studies. A large meta-analysis involving insulin-resistant subjects demonstrated QUICKI as a simple surrogate index with the best positive predictive power for determining development of diabetes. QUICKI provides a simple, useful, inexpensive, and minimally invasive surrogate for glucose clamp-derived measurements of insulin sensitivity and is appropriate and effective for use in research studies.11
The HOMA was first described in 1985 as a method for assessing beta cell function and insulin resistance from basal glucose and insulin or C-peptide concentrations.15 It derives an estimate of insulin sensitivity from the mathematical modelling of fasting plasma glucose and insulin concentrations. There is good correlation between estimates of insulin resistance derived from HOMA and from the euglycemic clamp.16 High HOMA scores denote low insulin sensitivity or high insulin resistance.
HOMA = (fasting serum insulin x fasting plasma glucose) / 22.5
Fasting serum insulin is expressed in μU/mL, and fasting plasma glucose in mmol/L.
Studies included randomized controlled trials examining the effect of flavanol-rich cocoa consumption on insulin sensitivity/resistance in overweight and obese individuals. Trials were included based on the following criteria: (a) investigating flavanol-rich cocoa products such as dark chocolate and cocoa beverages, (b) minimum treatment duration of two weeks, (c) random allocation to treatment and control group, (d) adult subjects with BMI greater than or equal to 25 kg/m2and (e) use of either QUICKI or HOMA as the outcome measure of insulin sensitivity. The mean change from baseline BMI in the control and intervention groups was defined as the secondary outcome. No restrictions were made with regard to gender, medication, baseline blood pressure, baseline blood glucose, risk profile, or comorbidities. Studies with parallel as well as crossover designs were included. No restrictions on the amount and preparation of treatment were made.
A literature search was done using the MeSH and PubMed databases and the Cochrane Library. The terms {"cacao"[MeSH Terms] OR "cacao"[All Fields] OR "chocolate"[All Fields]} AND {"insulin"[MeSH Terms] OR "insulin"[All Fields]} AND {"sensitivity"[All Fields]} AND {"humans"[MeSH Terms] AND Randomized Controlled Trial[ptyp] AND "adult"[MeSH Terms]} were entered on the PubMed search box. The terms “cocoa” and “insulin resistance” were searched in titles, abstracts or keywords in the Cochrane Central Register of Controlled Trials. Titles and abstracts resulting from the search strategies were independently screened by three investigators. Quality was assessed independently using guidelines published by the Cochrane Collaboration Clearly irrelevant titles or abstracts of articles were rejected on initial screening. The full texts of potentially relevant articles were reviewed to assess eligibility for inclusion in the meta-analysis. Any disagreement was resolved by discussion and consensus among the three investigators. Data were independently extracted by three reviewers using a standard data extraction form. Data were collected on study participants, intervention, control, outcomes, and potential effect modifiers such as trial duration, flavanol dose and preparation, gender, and comorbidities.
Three independent reviewers assessed the risk of bias as well as the quality of the trials included and created a risk of bias table according to the recommendations by the Cochrane Collaboration. Funnel plots were not created because of the limited number of trials included and the lack of studies with large sample sizes which may preclude a meaningful interpretation. The treatment effect was defined as the mean difference of the treatments in QUICKI, HOMA and BMI between the treatment and control groups. For continuous outcomes in the parallel study (Davison)17, the number of participants was assessed, and the means and standard deviations (SD) of the outcome measurement in the intervention and control arms were used. In crossover studies (Grassi8, Grassi18, Muniyappa19), it was intended that paired t test data would be extracted that evaluated whether the measurement on treatment minus the measurement on control for each subject was different from zero. However, because these data were rarely provided in practice, means and SDs separately on treatment and on control were used. This step provided a conservative estimate of effect and reduced the power of crossover studies to show real effects of interventions. The means and SDs of changes in the variables between baseline and the end of the intervention period (for the treatment and the control groups) were not provided hence the means and SDs of the outcome measurement in the treatment and control arms were used. Data not provided in numerical form were estimated from figures. The chi-squared test was used to assess whether observed differences in results are compatible with chance alone. I2 was used to quantify the degree of inconsistency among studies. Subgroup analyses were performed to explore heterogeneity. Data synthesis and statistical analyses were done using the Cochrane Collaboration Review Manager (RevMan, version 5.0.25). Investigation of heterogeneity was done using subgroup analyses by treatment preparation (chocolate bar versus cocoa drink) and treatment duration (2 weeks versus 12 weeks).
The study selection process is shown in Fig. 1. A total of 6 publications were retrieved from the electronic search. One publication was excluded on initial screening due to irrelevance of the title and abstract. In total, 5 publications were assessed in detail for inclusion. Four publications fulfilled the inclusion criteria (Table 1). One publication was excluded because the population included persons with normal BMI (Table 2).
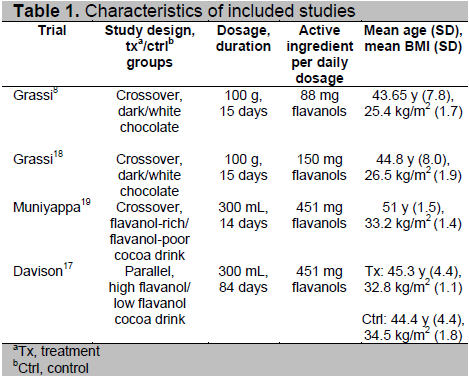
The 4 studies included in the meta-analysis were randomized controlled trials with insulin sensitivity/resistance as the primary outcome. Combining all studies, 118 individuals were analyzed in the QUICKI arm and 101 individuals in the HOMA arm. Two studies used both QUICKI and HOMA as the measure of insulin sensitivity. One study used only QUICKI and another study used only HOMA. Three of the 4 studies (Grassi 8,18, Muniyappa 19) used a crossover design and one study (Davison17) used a parallel design. The studies with crossover design incorporated a washout period of 7 days between the alternate treatment periods, then employed a 7-day run-in period before commencing with the treatments. The intervention period was a minimum of 2 weeks. Two studies (Grassi 8,18) used 100 g chocolate bars per day and two studies (Muniyappa 19, Davison 17) used 300 mL cocoa drink per day. Two studies involved overweight individuals and 2 studies also included obese individuals. One of the 4 studies included glucose intolerant subjects. Flavanol content varied widely between the studies, ranging from 88 to 451 mg/day (Table 1).
Click here to download Table 1
Table 1. Characteristics of included studies
One study20 was excluded because the population included persons with normal BMI. The characteristics of the excluded study are outlined in Table 2.
Adequate blinding of participants was not possible due to the obvious differences in appearance and taste among the trials using dark and white chocolate (as opposed to cocoa beverages). However, investigators and end point assessment were blinded in most studies. Other minor sources of potential bias included: (a) the absence of description of the methods of sequence generation in the randomization process and (b) insufficient data to permit judgment if participants or investigators could foresee the assignment to the treatment groups (allocation concealment). All of the trials included had insufficient data to rule out selective reporting
All studies involved random allocation of participants to either treatment or control group. Attempts to conceal allocation of intervention assignment were not stated in 3 studies. In one study (Grassi18), the involved physicians and staff were unaware of the group assignment. The patients did not receive information regarding the chocolate and were instructed not to disclose their assigned group to investigators.
In two studies involving cocoa and placebo drink preparations (Muniyappa19, Davison 17), study investigators and participants were blinded to treatment assignment. In one study (Grassi18), treatment involved chocolate doses rolled in aluminium foil and administered in dated, sequentially numbered, non-transparent boxes not labelled with regard to content.
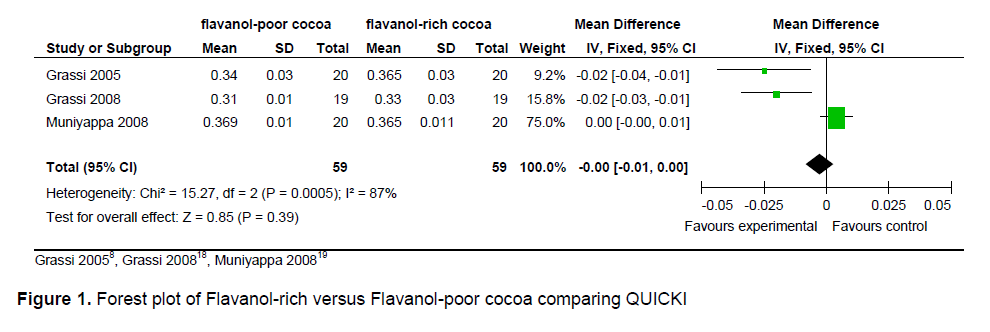
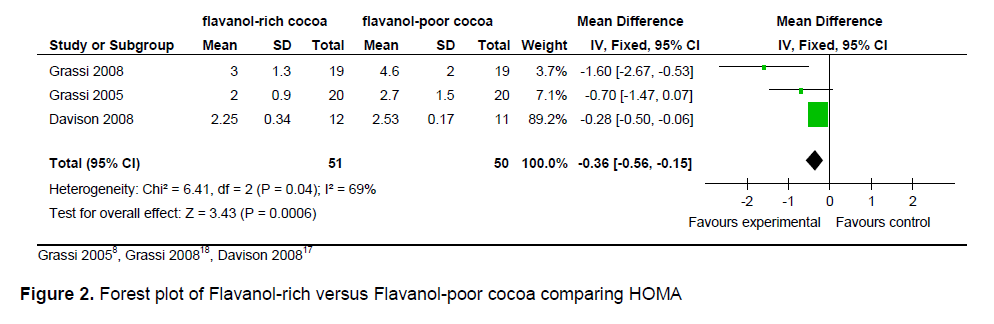
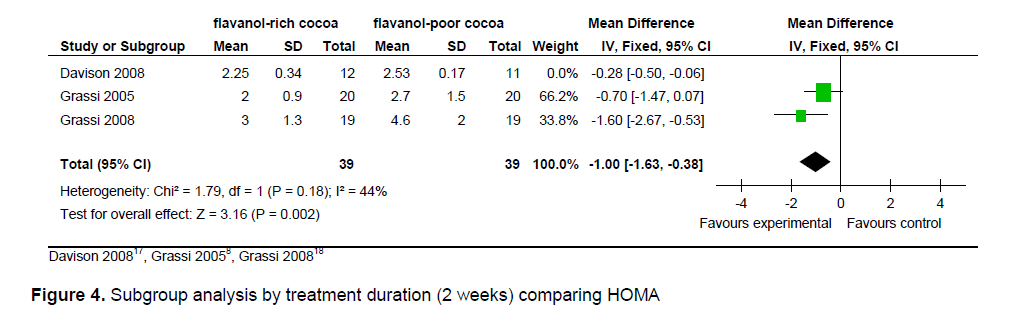
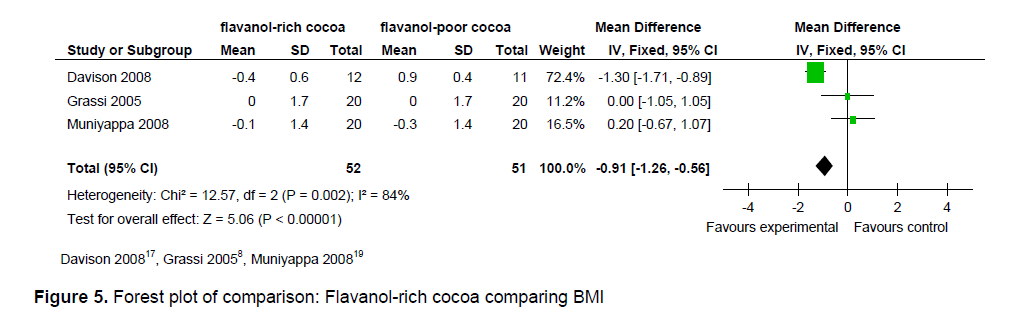
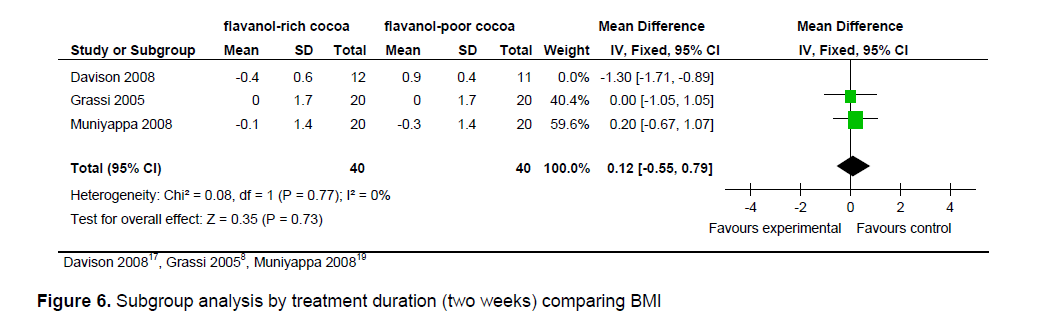
The statistical summary of results is shown in Table 3. Meta-analysis of 2 trial arms (QUICKI and HOMA) revealed that flavanol-rich cocoa is superior to flavanol-poor cocoa in increasing insulin sensitivity among overweight and obese individuals. However, a significant effect on increasing insulin sensitivity by flavanol-rich cocoa compared with control can only be seen in the HOMA arm [mean QUICKI: -0.00 (95% CI, -0.01, -0.00), p = 0.39; mean HOMA: -0.36 (95% CI, -0.56, -0.15), p = 0.0006] (Figures 2 and 3). Flavanol-rich cocoa also significantly decreased BMI (Figure 6). Heterogeneity between trials was high (QUICKI: I2 = 87%, HOMA: I2 = 69%, BMI: I2 = 84%), prompting subgroup meta-analysis (Figures 4, 5 and 7). For subgroup analysis by treatment preparation, trials using flavanol-rich chocolate bars were pooled. A significant increase in insulin sensitivity measured by QUICKI can be seen in the flavanol-rich group, with loss of the previously noted heterogeneity [mean QUICKI: -0.02 (95% CI, -0.03, -0.01), p = 0.0002; I 2 = 0%]. For subgroup analysis by treatment duration, trials involving 2 weeks of treatment were pooled. Flavanol-rich cocoa significantly increased insulin sensitivity measured by HOMA compared with flavanol-free cocoa, with a decrease in the previously noted heterogeneity [mean HOMA: -1.00 (95% CI, -1.63, -0.38), p = 0.002; I2 = 44%].
Four trials on the effects of flavanol-rich cocoa products (as dark chocolate and cocoa beverages) on insulin sensitivity/resistance in overweight and obese individuals were included in this review. The principal finding of this
meta-analysis is an increase in insulin sensitivity with intake of flavanol-rich cocoa. The precise mechanisms responsible for the presumed effect on increasing insulin sensitivity by flavanol-rich cocoa are not fully explored. However, an increase in vasodilating nitric oxide, which improves endothelial function, and thus increases insulin sensitivity, is considered a likely pathway. Intake of flavanol-rich cocoa also significantly reduced BMI which may explain the increase in insulin sensitivity found in this review.
Click here to download Figure 1
Figure 1. Forest plot of Flavanol-rich versus Flavanol-poor cocoa comparing QUICKI
Click here to download Figure 2
Figure 2. Forest plot of Flavanol-rich versus Flavanol-poor cocoa comparing HOMA
Click here to download Figure 3
Figure 3. Subgroup analysis by treatment preparation (chocolate bar) comparing QUICKI
Click here to download Figure 4
Figure 4. Subgroup analysis by treatment duration (2 weeks) comparing HOMA
Click here to download Figure 5
Figure 5. Forest plot of comparison: Flavanol-rich cocoa comparing BMI
Click here to download Figure 6
Figure 6. Subgroup analysis by treatment duration (two weeks) comparing BMI
Most of the trials in this review used a crossover study design. This is suitable for evaluating an intervention with a temporary effect in the treatment of stable, chronic conditions such as insulin sensitivity/resistance. The main problem associated with cross-over trials is that of carry-over, where the effects of an intervention given in one period may persist into a subsequent period. The washout period incorporated in the trials was an effort to reduce carry-over. Given the short half-life of cocoa flavanols, the one week washout period was sufficient to minimize carry-over effects.
Several limitations were identified. The studies displayed a diverse spectrum of treatment regimens. The statistical heterogeneity between the trials found in the analysis likely reflects the clinical diversity in treatment regimens, so that subgrouping by duration or preparation reduced apparent levels of heterogeneity. Estimated daily flavanol intake varied widely across studies. Treatment duration ranged from 2 to 12 weeks. Another limitation is the lack of an adequate control substance since white chocolate, instead of flavanol-free chocolate, was used in most trials. In addition, when there is evidence of effectiveness of flavanol-rich cocoa, it is not clear whether the flavanols themselves, rather than the bioactive components, were solely or partially responsible for the observed effects. The weaknesses of the available data include few and very small studies and the mean percent change from baseline of the outcome measures were not reported.
The meta-analysis suggests that flavanol-rich cocoa is superior to flavanol-poor cocoa in increasing insulin sensitivity among overweight and obese individuals. However, significant statistical heterogeneity across studies were found, and questions pertaining to the appropriate preparation, dose and treatment duration warrant further investigation before cocoa products can be recommended for this purpose.
There is no commercially available cocoa with high flavanol content because of extensive processing. In contrast to chocolate, natural cocoa powder is prepared by squeezing out fat. It retains all the flavor of chocolate but is much lower in calories.21 Nonetheless, it is important to note that caution is always warranted when considering dietary recommendations for daily intake of cocoa products with significant energy, fat, and sugar content potentially leading to adverse metabolic effects.
The findings of this review support a potentially beneficial action of flavanol-rich cocoa on insulin sensitivity in overweight and obese individuals and suggest directions for further research in this area. The use of homogeneous treatment regimens and/or using blinded studies may be warranted in future clinical trials. In addition, longer treatment duration may be reasonable to accurately assess insulin sensitivity as well as likely adverse effects of prolonged cocoa intake.
1. Kahn BB, Flier JS. Obesity and insulin resistance. J Clin Invest 2000;106(4):473-81.
2. Singhal A. Endothelial dysfunction: Role in obesity-related disorders and the early origins of CVD. Proc Nutr Soc 2005;64(1):15-22.
3. Sydow K, Mondon CE, Cooke JP. Insulin resistance: Potential role of the endogenous nitric oxide synthase inhibitor ADMA. Vasc Med 2005;10(Suppl1):S35-43.
4. Petrie JR, Ueda S, Webb DJ et al. Endothelial nitric oxide production and insulin sensitivity: A physiological link with implications for pathogenesis of cardiovascular disease. Circulation 1996;93(7):1331-3.
5. Campia U, Panza JA. Flavanol-rich cocoa a promising new dietary intervention to reduce cardiovascular risk in type 2 diabetes? J Am Coll Cardiol 2008;51(22):2150-2.
6. Hooper L, Kroon PA, Rimm EB et al. Flavonoids, flavonoid-rich foods, and cardiovascular risk: A meta-analysis of randomized controlled trials. Am J Clin Nutr 2008;88(1):38-50.
7. Desch S, Schmidt J, Kobler D et al. Effect of cocoa products on blood pressure: Systematic review and meta-analysis. Am J Hypertens 2010;23(1):97-103.
8. Grassi D, Lippi C, Necozione S et al. Short-term administration of dark chocolate is followed by a significant increase in insulin sensitivity and a decrease in blood pressure in healthy persons. Am J Clin Nutr 2005;81(3):611–4.
9. Almoosawi S, Fyfe L, Ho C et al. The effect of polyphenol-rich dark chocolate on fasting capillary whole blood glucose, total cholesterol, blood pressure and glucocorticoids in healthy overweight and obese subjects. Br J Nutr 2010;103(6):842-50.
10. Faridi Z, Njike VY, Dutta S et al. Acute dark chocolate and cocoa ingestion and endothelial function: A randomized controlled crossover trial. Am J Clin Nutr 2008;88(1):58-63.
11. Katz A, Nambi SS, Mather K et al. Quantitative insulin sensitivity check index: A simple, accurate method for assessing insulin sensitivity in humans. J Clin Endocrinol Metab 2000;85(7):2402-10.
12. Wallace TM, Levy JC, Matthews DR. Use and abuse of HOMA modelling. Diabetes Care 2004;27(6):1487-95.
13. DeFronzo RA, Tobin JD, Andres R. Glucose clamp technique: A method for quantifying insulin secretion and resistance. Am J Physiol 1979;237(3):E214-23.
14. Muniyappa R, Lee S, Chen H et al. Current approaches for assessing insulin sensitivity and resistance in vivo: Advantages, limitations, and appropriate usage. Am J Physiol Endocrinol Metab 2008;294(1):E15-26.
15. Matthews DR, Hosker JP, Rudenski AS et al. Homeostasis model assessment: Insulin resistance and B-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985;28(7):412-9.
16. Bonora E, Targher G, Alberiche M et al. Homeostasis model assessment closely mirrors the glucose clamp technique in the assessment of insulin sensitivity: Studies in subjects with various degrees of glucose intolerance and insulin sensitivity. Diabetes Care 2000;23(1):57-63.
17. Davison K, Coates AM, Buckley JD et al. Effects of cocoa flavanols and exercise on cardiometabolic risk factors in overweight and obese subjects. Int J Obes (Lond.) 2008;32(8):1289-96.
18. Grassi D, Desideri G, Necozione S et al. Blood pressure is reduced and insulin sensitivity increased in glucose-intolerant, hypertensive subjects after 15 days of consuming high-polyphenol dark chocolate. J Nutr 2008;138(9):1671-6.
19. Muniyappa R, Hall G, Kolodziej TL et al. Cocoa consumption for 2 wk enhances insulin-mediated vasodilatation without improving blood pressure or insulin resistance in essential hypertension. Am J Clin Nutr 2008;88(6):1685-96.
20. Grassi D, Lippi C, Necozione S et al. Short-term administration of dark chocolate is followed by a significant increase in insulin sensitivity and a decrease in blood pressure in healthy persons. Am J Clin Nutri 2005;81:611-4.
21. Hollenberg NK, Fisher ND. Is it the dark in dark chocolate? Circulation 2007;116(21):2360-2.
Authors are required to accomplish, sign and submit scanned copies of the JAFES Declaration that the article represents original material that is not being considered for publication or has not been published or accepted for publication elsewhere.
Consent forms, as appropriate, have been secured for the publication of information about patients; otherwise, authors declared that all means have been exhausted for securing such consent.
The authors have signed disclosures that there are no financial or other relationships that might lead to a conflict of interest. All authors are required to submit Authorship Certifications that the manuscript has been read and approved by all authors, and that the requirements for authorship have been met by each author.