Anti-thyroid drugs are the first line therapy for treatment of hyperthyroidism since their discovery in 1941.[1] The ATDs are associated with a variety of minor side effects as well as potentially life-threatening complications such as agranulocytosis.[2] Agranulocytosis is defined as an ANC of less than 500 cells/μL or 0.5 x 10^9, which carries a very high risk for infection.[2][3] The occurrence of ATD-induced agranulocytosis is uncommon, in less than 0.1% to 0.5% of hyperthyroid patients on either thiamazole or propylthiouracil.[1][2] It has an incidence rate of 3 in every 10,000 patients per year.[4] In 2008, agranulocytosis was also found in 0.37% and 0.35% of hyperthyroid patients receiving propylthiouracil (PTU) and methimazole (MMI), respectively.[5] Although ATD-induced agranulocytosis is not commonly observed in hyperthyroid patients, it has a high mortality rate of 21.5% worldwide.[6] Among the drugs that can cause agranulocytosis, ATDs are among the medications with the highest relative risks of causing agranulocytosis, along with with sulfasalazine and co-trimoxazole.[7]
The common ATDs used in the Philippines are MMI and PTU. Carbimazole, a precursor of MMI, is also widely used. It is rapidly converted to MMI in the serum and has the same mechanism of action.[8] In 2011, the American Association of Clinical Endocrinologists and the American Thyroid Association explained in their management guidelines that MMI should be used in virtually every patient who chooses ATD for Graves’ disease (GD), except only during the first trimester of pregnancy, because it has the benefit of a once daily administration and a reduced risk of major side effects compared to PTU.[8] Also, the side effects of MMI are dose-related, whereas those of PTU are less clearly related to the dose. Minor side effects such as cutaneous reactions (usually urticaria or macular rashes), arthralgia and gastrointestinal upset occurred in approximately 5% of patients, with equal frequency for both drugs. Cross-reactivity between the 2 agents may be as high as 50%.[2] PTU may rarely cause agranulocytosis, and low doses of MMI may be less likely.[8] Another important finding is that agranulocytosis may still occur after a previous uneventful course of ATD, as re-exposure to the ATD may occur when patients have a relapse and resume a second course of treatment.[2] The exact mechanism of ATD-induced agranulocytosis is still undetermined, but it is assumed to be related to autoimmunity as demonstrated by anti-granulocyte antibodies seen using immunofluorescence and cytotoxicity assays.[2]
A recent advancement on genetics showed 2 risk genotypes (HLA-B*38:02 and HLA-DRB1*08:03) that have independent association with ATD-induced agranulocytosis by human leukocyte antigen (HLA) genotyping and genome-wide association study (GWAS).[9] A study on GD included 42 patients with ATD-induced agranulocytosis and 927 with normal white blood cell count. HLA-B*38:02 was found in 59.52% of ATD-induced agranulocytosis, but only in 6.41% of those who did not experience agranulocytosis. HLA-DRB1*08:03 was seen in 52.38% of ATD-induced agranulocytosis, compared to only 15.22% of control patients. Although this is the largest research on the genetics of ATD-induced agranulocytosis with 2-stage study design, the researchers recommended to conduct another study using the same or other populations to support the validity of the result.[9]
Several studies have described the clinical characteristics of ATD-induced agranulocytosis. It was found to be more common in elderly patients using MMI in dosages more than 40 mg/day, while the prevalence with PTU was not dose-dependent.[10] Most cases were observed by to occur within 3 months following ATD initiation.[11] However, another study on a Japanese population found that the occurrence of ATD-induced agranulocytosis was not associated with dose, age and treatment duration.[12] Age, sex and type of ATD were not associated with any significant difference in the occurrence of agranulocytosis based on local data.[13]
In 1990, granulocyte colony-stimulating factor, a recombinant human hematopoietic growth factor used to decrease infection associated with neutropenia caused by the myelosuppression from chemotherapy of cancer patients, was found to improve the ANCs of some patients with ATD-induced agranulocytosis.[14] It is a hematopoietic growth factor that acts on hematopoietic cells to stimulate production, maturation and activation of neutrophils. It also increases migration and cytotoxicity of neutrophils. The actions of GCSF include stimulation and differentiation of myeloid granulocyte progenitors, squeezing mature granulocytes from bone marrow and shifting neutrophils from the perivascular to the vascular space.[15]
Studies on the benefits of GCSF on ATD-induced agranulocytosis are mostly non-randomized and retrospective because of its low incidence rate. A randomized prospective study done in 1999 studied 24 GD patients with ATD-induced agranulocytosis in Japan: 14 patients were given GCSF and 10 were controls.[15] The objective was to examine whether GCSF was effective for ATD-induced agranulocytosis. They defined agranulocytosis as ANC less than 500/mm3, and agranulocytosis recovery time as number of days required for ANC to exceed 500/mm3. The mean age and ANC were not statistically significant between the 2 groups. Recovery time in the GCSF-treated group did not differ from that of the untreated group in those patients with moderate and severe agranulocytosis. It was concluded that GCSF was generally ineffective for ATD-induced agranulocytosis.[15]
In 2001, contrary findings were reported by a retrospective cohort study done in France. The mean duration of hematological recovery (6.8 ± 4 days for GCSF versus 11.6 ± 5 days for non-GCSF), mean treatment duration of antibiotic therapy (7.5 ± 3.8 days versus 12 ± 4.5 days) and length of hospitalization (7.5 ± 3.8 days versus 12 ± 4.5 days) were significantly reduced with GCSF.[16] The different findings of the Japanese study by Fukata and colleagues were attributed to the use of inappropriate GCSF doses at 100 to 200 μg/day subcutaneously and the absence of a well-defined target population.[16] On the other hand, the French study observed the effect of 300 μg/day of subcutaneous GCSF in a well-defined population.
A retrospective study done on 109 patients with ATDinduced agranulocytosis by Tajiri and colleagues in 2005 designated patients as symptomatic and asymptomatic.[14] GCSF therapy was found to shorten the period of recovery from ATD-induced agranulocytosis in asymptomatic patients and symptomatic patients with ANC above 0.1 x 10^9/L, and not those with symptoms and ANC below 0.1 x 10^9/L.[14]
A recent retrospective cohort study done by Watanabe and colleagues in 2012 involved 55 patients with ATD-induced agranulocytosis alone (n=50) and with pancytopenia (n=5). GCSF was given in 35 patients with agranulocytosis alone and in 2 with pancytopenia. The remaining received either steroids (n=10) or supportive care (n=8 patients). Of those with agranulocytosis alone, the recovery time was 7 days (range, 2 to 22 days) in the GCSF group, 9 days (5 to 11 days) in those given steroids, and 9 days (4 to 21 days) for supportive care alone. Fifty-four patients treated with GCSF recovered significantly earlier (7 days, range 2 to 22 days) than those who did not receive GCSF (9 days, 4 to 21 days) (p=0.004).1
A local retrospective analysis done in 2008 by Macaballug studied the severity of ATD-induced agranulocytosis in 14 patients. Two out of the 14 patients were given GCSF. Doses of PTU greater than 300 mg/day were correlated with agranulocytosis. The ANCs of patients with normal BMI were higher compared to those who were underweight, but the difference was not statistically significant. A mean ANC less than 24 was correlated with poor outcome. The use of GCSF improved the outcome in the 2 patients with ATD-induced agranulocytosis.[17]
The benefits of GCSF on ATD-induced agranulocytosis do not seem to be consistent across studies.[14]-[20] In studies done by Watanabe, Andres and Tamai, GCSF shortened the recovery period from agranulocytosis.[1][16][18][19] GCSF was ineffective against ATD-induced agranulocytosis in a prospective controlled study.[15]
The cost of GCSF is also an important issue for both the patient and physician, particularly in emerging countries such as the Philippines. GCSF is an expensive treatment modality. With the recommended dose of GCSF at 5 μg/kg/day, this typically requires an average of 300 μg per 1 mL vial injected subcutaneously once a day.[21] The physician’s decision to treat rests on careful assessment of benefit from treatment.
In 2006, the American Society of Clinical Oncology (ASCO) provided updated recommendations for GCSF use in non-cancer or non-chemotherapy-related neutropenic patients. The ASCO recommended that GCSF should not be routinely used as adjunctive treatment with antibiotic therapy for patients with fever and neutropenia. They emphasized that GCSF should be considered in patients with fever and neutropenia who are at high-risk for infection-associated complications, or who have prognostic factors that are predictive of poor clinical outcomes. High-risk features include expected prolonged (more than 10 days) and profound (less than 0.1 x 10^9/L) neutropenia, age older than 65 years, uncontrolled primary disease, pneumonia, hypotension and multiorgan dysfunction (sepsis syndrome), invasive fungal infection, or being hospitalized at the time of the development of fever.[21] The prognostic factors for poor clinical outcomes can be used in considering GCSF treatment, since there is no existing standard of care, clinical practice guideline nor consensus specifically for ATD-induced agranulocytosis at this time. These high-risk features were included in the inclusion criteria so that all the patients in this study have similar indications for recommending GCSF therapy.
Given the lack of evidence on the efficacy of GCSF in drug-induced neutropenia and agranulocytosis for nononcologic conditions and the paucity of local data, its utility and practicality in our setting has yet to be established.2 This study sought to present data from our institution to describe the clinical characteristics of patients who had anti-thyroid drug-induced agranulocytosis, and to determine if there is a significant difference in the recovery time and duration of hospital stay of patients with with and without GCSF therapy.
METHODOLOGYStudy Design
A retrospective cohort study was performed.
Inclusion and Exclusion CriteriaAdmitted patients with diagnosed hyperthyroidism on anti-thyroid medication, age 18 years old and older, documented temperature ≥37.8 °C, ANC upon admission below 500 cells/μL or below 0.5 x 10^9/L, with at least one of the prognostic factors predictive of poor clinical outcomes where GCSF administration can be considered [age greater than 65 years old, uncontrolled primary disease (uncontrolled hyperthyroidism), expected prolonged (more than 10 days) and profound (less than 0.1 x 10^9/L) neutropenia, pneumonia fungal infection, hypotension, sepsis or multi-organ dysfunction] were included. Patients being worked-up for or previously diagnosed with anemia; with hematologic disorder, malignancy, history of chemotherapy and radiation therapy; or on treatment with methotrexate, cyclophosphamide, colchicine, azathioprine, ganciclovir, clozapine, sulfasalazine, amodiaquine, deferiprone, dapsone, dypirone, penicillin G, rituximab, sulfasalazine, ticlopidine, anti-arrhythmic drugs such as tocainide, procainamide and flecainide were excluded.
Sample SizeThe sample size was calculated using the computation for difference between 2 means, with the level of significance and power set at 0.05 (Zα=1.96) and 80% (Zβ=0.84), respectively. Values for the difference in mean and standard deviation were based on The Medical City's census on recovery days between patients who were given GCSF therapy and patients who were not given GCSF therapy from January 2005 to June 2014, wherein D=8.466 and σ=7.5. The sample size computed for each group was 14 (N=28).
Study ProcedurePrior to data collection, the study was reviewed and approved for implementation by the Institutional Review Board of The Medical City. The Department of Medicine census and the Medical Records Department database were used to search the terms hyperthyroidism, Graves’ disease, agranulocytosis, neutropenia and febrile neutropenia in the diagnosis of patients admitted from January 1, 2005 to June 30, 2014. Clinical characteristics such as age, sex, BMI, type and duration of anti-thyroid drug use, ANCs and their dates upon admission and upon reaching 500/μL or above, and use of antibiotics and/or steroids were obtained. Patients were classified into two groups according to treatment or non-treatment with GCSF. Clinical outcomes taken were recovery time from agranulocytosis (number of days from the date of ANC upon admission until ≥500/microL was reached) and the duration of hospital stay (excluding the days needed for the RAI treatment) were compared between the two groups.
Data AnalysisData were analyzed using Stata® version 11. Continuous variables were described using median and range. Categorical variables were expressed as frequency and percentage. Comparison of age, BMI, duration of ATD use, ANC level upon admission, recovery time and duration of hospital stay between the two groups were analyzed using Mann-Whitney U-test, assuming that the difference across samples were not normally distributed. Categorical variables were analyzed using Fisher exact test. Significance was set at 0.05.
Ethical ConsiderationsThis manuscript has been duly approved by the Institutional Review Board of The Medical City.
A total of 30 medical records of patients with anti-thyroid drug-induced agranulocytosis admitted in from January 2005 to June 2014 were reviewed. Two patients were excluded: one due to concomitant anemia, while the other had no prognostic factor predictive of poor clinical outcome. Of the remaining 28 patients, 14 patients were given GCSF therapy and the remaining 14 patients were not given GCSF.
The median ages were 39.5 and 28 years for the GCSF and non-GCSF group, respectively. Age (p=0.197), sex (p=1.0) and BMI (p=0.826) were not significantly different between the 2 groups (Table 1). Thirteen patients (93%) were on imidazoles (either methimazole or carbimazole) and one (7%) on PTU in each of the GCSF and non-GCSF groups. The shortest duration of ATD use prior to the development of agranulocytosis was 2 weeks in the GCSF group, compared to one week in the non-GCSF. The longest duration of ATD use was 36 weeks in the GCSF group and 16 weeks in the non-GCSF. The median duration of ATD was 5 weeks and 4 weeks for the GCSF and the non-GCSF groups, respectively, with no significant difference (p=0.424). The lowest ANC was 14 cells/μL in the GCSF group and 65 cells/μL in the non- GCSF. The median ANC was lower in the GCSF group (62.5 cells/μL) than the non-GCSF (258 cells/μL) (p=0.002).
Click here to download Table 1Table 1. Clinical characteristics of patients with anti-thyroid drug-induced agranulocytosis
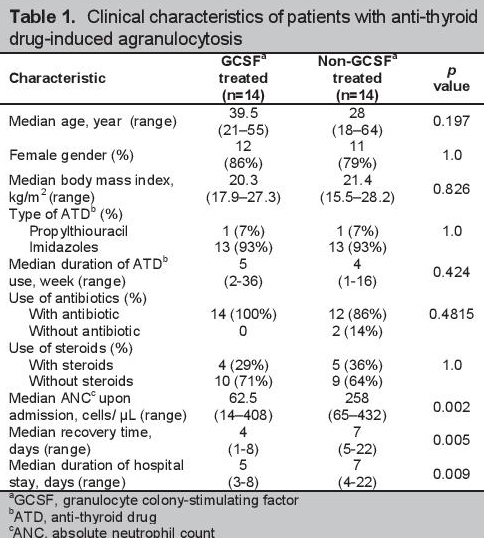
All patients in the GCSF group were given antibiotics compared to only 12 (86%) in the non-GCSF group. Steroids were given in 4 patients (29%) in the GCSF group, and 5 (36%) in the non-GCSF. There was no significant difference in the number of patients treated or untreated with antibiotics and steroids, with a p value of 0.4815 and 1.0, respectively.
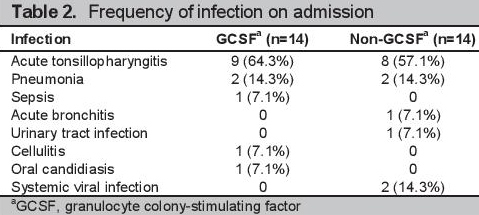
The most common infection in both groups was acute tonsillopharyngitis (64.3% in the GCSF, 57.1% in the non-GCSF) (Table 2). Thirteen of the 14 patients had confirmed uncontrolled hyperthyroidism in each of the GCSF and non-GCSF groups. The remaining patients with undocumented thyroid function had pneumonia. Both uncontrolled hyperthyroidism and pneumonia were prognostic factors for poor clinical outcomes.
Click here to download Table 2Table 2. Frequency of infection on admission
There was a significantly shorter recovery time in the GCSF compared to the non-GCSF group (4 versus 7 days, p=0.005). Duration of hospital stay was significantly shorter in the GCSF-treated group (5 versus 7 days, p=0.009), despite the significantly lower ANC level on admission (Table 1).
Clinical features, such as ANC less than 500 cells/μL or less than 0.5 x 109/L and high risk for infection, were similar in all the included patients. These were also found in the studies of Fukata and Andres.[15][16] With the exception of ANCs upon admission, there were no significant differences between the GCSF-treated and nontreated groups in all of the characteristics, particularly age (p=0.197), sex (p=1) and BMI (p=0.826), type (p=1) and duration (p=0.424) of ATD, use of antibiotic (p=0.4815) and steroid treatment (p=1). The predominantly female composition of both groups was consistent with studies on ATD-induced agranulocytosis done in Japan, France and the Philippines.[1][14]-[17] This may be attributed to the 1:5 male to- female ratio of hyperthyroidism worldwide.[22]
Most of the hyperthyroid patients who developed antithyroid drug-induced agranulocytosis were on imidazoles, as only a small proportion were on PTU.[1][14]-[16] Imidazoles are preferred over PTU due to their ability to rapidly achieve euthyroidism, once daily dosing assuring better compliance and less reported toxicity.[23]
The most common initial adverse reactions to ATDs are pruritus and rashes. This can occur within 24 hours after taking the anti-thyroid drug. Agranulocytosis, the more life-threatening side effect, is usually seen within 2 months of anti-thyroid drug use.[1] The median duration of ATD use was not significantly different between the two groups (5 weeks in the GCSF versus 4 weeks in the non-GCSF, p=0.424).
In our study, all patients were febrile with ANC less than 500 cells/μL. The ASCO recommends against the routine use of GCSF in febrile neutropenia.[21] Its use should be considered, however, in patients with fever and neutropenia who are at high-risk for infection-associated complications, or who have prognostic factors that are predictive of poor clinical outcomes. These prognostic factors were in our inclusion criteria to ensure that the patients in both groups were all candidates for GCSF therapy. High-risk features include expected prolonged (more than 10 days) and profound (less than 0.1 x 10^9/L) neutropenia, age greater than 65 years, uncontrolled primary disease (uncontrolled hyperthyroidism documented in 26 patients), pneumonia (2 patients each in GCSF and non-GCSF groups), fungal infection (1 patient), hypotension and multi-organ dysfunction or sepsis syndrome (1 patient) or being hospitalized at the time of the development of fever.[21] Two patients with undocumented hyperthyroidism had pneumonia. All of the patients in the 2 groups have at least one prognostic factor predictive of poor clinical outcomes.
Antibiotics and steroids were considered as supportive management for ATD-induced agranulocytosis. Antibiotic use was described but not correlated to GCSF treatment in the studies of Watanabe, Andres and Macaballug.[1][16][17] The studies generally stated that antibiotics and steroids improved the prognosis of the ATD-induced agranulocytosis, without reference to its effect on recovery time and duration of hospital stay in the GCSF or non-GCSF group. Glucocorticoids are thought to have 3 mechanisms for causing neutrophilic granulocytosis: (1) induction of detachment of neutrophils from the endothelial lining of blood vessels, (2) delay in migration of neutrophils from circulation into tissues, and (3) improvement of neutrophil survival by suppression of apoptosis.[24] In our study, there was a balance between patients given antibiotic and steroids in the GCSF and non-GCSF groups, somewhat reducing their confounding effects.
Fukata and colleagues found that the recovery time in the GCSF-treated group did not differ from the untreated when ANCs were less than 500 cells/μL.[15] The disparity with our findings may be due to the different dosage of GCSF (300 μg/day) given in our institution, as per the current recommended dose of 5 μg/kg body weight/day.[21] The significantly shorter recovery time (p=0.005) and duration of hospital stay (p=0.009) despite lower ANC levels of the GCSF group in this study was consistent with the findings of Andres and colleagues.[16]
GCSF significantly shortened the recovery time and duration of hospitalization of hyperthyroid patients with ATD-induced agranulocytosis. Physicians may use GCSF in patients with anti-thyroid drug-induced agranulocytosis who have prognostic factors that are predictive of poor clinical outcomes.
RecommendationThe generalizability of the results is limited by the reported experience in a single tertiary level hospital. Collaborative research with other institutions to compare clinical experiences may help initiate the formulation of evidence-based local guidelines for the care of patients with anti-thyroid drug-induced agranulocytosis.
AcknowledgementsThe authors express their appreciation to the staff of the Medical Information Department of The Medical City for their assistance in the retrieval of medical records, and to the Pharmacy Department for providing the essential data on GCSF in their In- Patient Drug Dispensing System.
Statement of AuthorshipAll authors have given approval to the final version submitted.
Author DisclosureAll the authors have declared no conflict of interest to the work carried out in this paper.
Funding SourceNone.
[1] Watanabe N, Narimatsu H, Noh JY, et al. Antithyroid drug-induced hematopoietic damage: A retrospective cohort study of agranulocytosis and pancytopenia involving 50,385 patients with Graves’ disease. J Clin Endocrinol Metab. 2012;97(1):E49-53. DOI