The estimated global prevalence of diabetes is 8.8%, with the majority living in low to middle income countries.[1] In 2013, the prevalence of diabetes in the Philippines among adults 20 years and above was at 5.4% and is increasing.[2] Persons with diabetes are predisposed to chronic infections and inflammation of the oral tissues, including periodontal diseases, which cause substantial oral functional disability.[3] Periodontitis has been considered as the sixth complication of diabetes.[4] It is a complex disease with numerous causal risk factors (including diabetes), characterized by the loss of connective tissues within the periodontium and the destruction of alveolar bone support.[5]
Recently, much has been published about periodontitis and its relationship with diabetes and vice-versa. In diabetes, the production of advanced glycated endproducts (AGEs), which activate host cells such as monocytes/macrophages and endothelial cells, may lead to the release of pro-inflammatory cytokines and proteases which damage the gingival tissues and cause resorption of alveolar bone.[6] Studies have identified that the risk of periodontitis was 3 to 4 times higher in people with diabetes.[5][7] Susceptibility to periodontitis is increased with poor glycemic control and more severe forms of periodontitis are observed in those with poorly controlled diabetes.[5][8][9] The prevalence of periodontitis among Filipinos with type 2 diabetes mellitus (T2DM), 35 years old and above is observed to be as high as 68-94%.[10][11]
There is convincing evidence that there is a bidirectional association between periodontitis and diabetes.[6][7] Diabetes and periodontal disease share a common pathway in inflammation resulting in increased levels of inflammatory mediators that can further increase insulin resistance.[12] Periodontal infection increases systemic inflammation by contributing to the cycle of hyperglycemia and AGEs binding accumulation thus the tendency for increasing the risk of developing diabetes or further increasing glycated hemoglobin (A1c) levels.[7] An improvement in glycemic control has been shown with early detection and treatment of periodontitis in both controlled and uncontrolled diabetes.[13]-[16]
Although prevalent, periodontitis is generally hidden, thus there is a need for routine oral evaluation in persons with diabetes.[7] While local and international guidelines recommend routine clinical screening and early referrals to dentistry, oral health awareness is lacking in the Philippines.[17] There is also a lack of access to public health care and the majority pay a larger out-of-pocket share.[1] Persons with diabetes have a higher utilization of dental procedures and may benefit from increased frequency of prophylactic services.[18] However, they are hesitant to see the dentist and probable reasons for not consulting is the cost of dental treatment as medications alone account for much of the patient’s budget.[17] Thus, there is a need to develop strategies to promote prevention and control of periodontitis in settings were income is limited.
Untreated serious periodontitis (moderate to severe periodontitis) is associated with tooth loss and progression of pocket depths.[19] In those who underwent periodontal treatment, an initial pocket probe depth (PPD) range of 4-6 mm was a risk factor for tooth loss.[20][21] It is also recognized that due to untreated or inadequately controlled serious periodontitis, the systemic inflammatory burden may also be increased.[22]
The Centers for Disease Control and Prevention (CDC) in collaboration with the American Academy of Periodontology (AAP) has formulated self-report questionnaires that appear to be promising in predicting the prevalence and severity of periodontitis among the adult population.[23]-[25] These validated self-reported oral health questions were translated into Filipino and together with other relevant oral health, medical and demographic variables; multivariate logistic regression analyses were done to determine predictors of serious periodontitis.[11] Predictors of serious periodontitis among adult Filipinos with diabetes were low education status, tooth loss >6, poor gum health, history of loose teeth and poor tooth appearance. With these, revised questions in English as well a Filipino version and a scoring system predictive for serious periodontitis were formulated.
Currently, this questionnaire has not been validated and there are no other validated clinical oral health screening questionnaires available locally. The validation of such a questionnaire would be useful in our setting in which resources are limited and thus would lessen the costs of screening. This paper aims to do the following:
- To determine the validity of the Oral Health Screening Questionnaire for Persons with Diabetes in estimating the prevalence of serious periodontitis.
- To compute the cut off value for the Oral Health Screening Questionnaire for Persons with Diabetes that is most predictive for serious periodontitis using a receiver operator curve (ROC).
Design/Setting
This is a cross-sectional criterion referenced study that was conducted at the tertiary outpatient clinics of the University of the Philippines - Philippine General Hospital (UP-PGH). The study was reviewed by the University of the Philippines Manila Research Ethics Board (UPMREB) Panel prior to commencement. The study participants were recruited consecutively from September 2015 – January 2016.
Study SampleUsing Epi Info version 7, the minimum sample size requirement was estimated to be at least 138 based on an estimated sensitivity of 90% (unpublished data) by Lo et al., alpha (α) = 5%, and a margin of error = 5%.[11] The computed 138 minimum sample size was increased to 173 accounting for possible 20% non-response.
Selection Criteria Inclusion Criteria- Adult Age ≥35 years) Filipino diagnosed with type 2 diabetes mellitus for at least 1 year. Diagnosis of type 2 diabetes was based on the American DiabetesAssociation criteria as follows:[26]
- Fasting Blood Sugar (FBS) ≥126 mg/dl on 2 determinations;
- Symptoms of hyperglycemia and Random Blood Sugar (RBS) ≥200 mg/dl;
- 2-hour plasma glucose ≥200 mg/dl after a 75 grams Oral Glucose Tolerance Test (OGTT);
- Standardized A1c ≥6.5%
- Dentulous persons with ≥6 teeth present
- Recent A1c result done within the past 3 months
- Able to read, comprehend and respond to the series of questions
- Willing to undergo a dental examination
- Patients with heart murmurs that would require antibiotics prior to dental examination
- Inclusion is voluntary. Withdrawal is allowed should the patient decide to stop participating even if consent was already given.
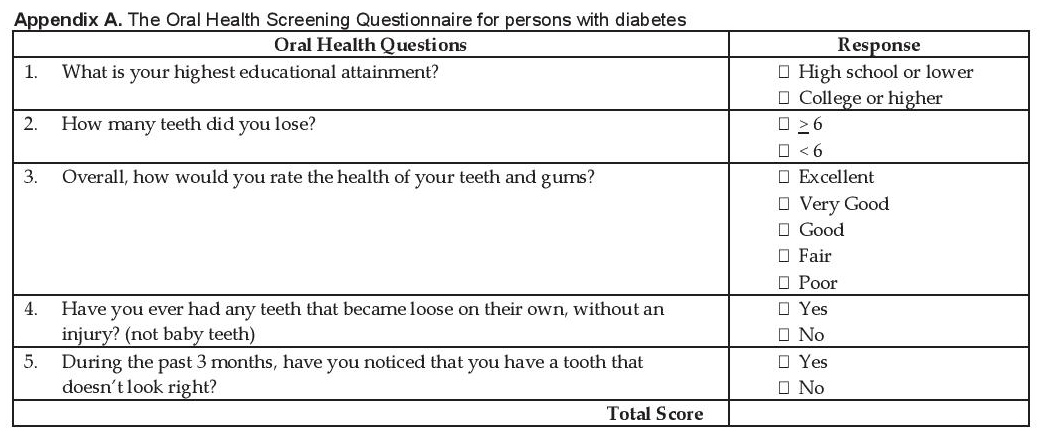
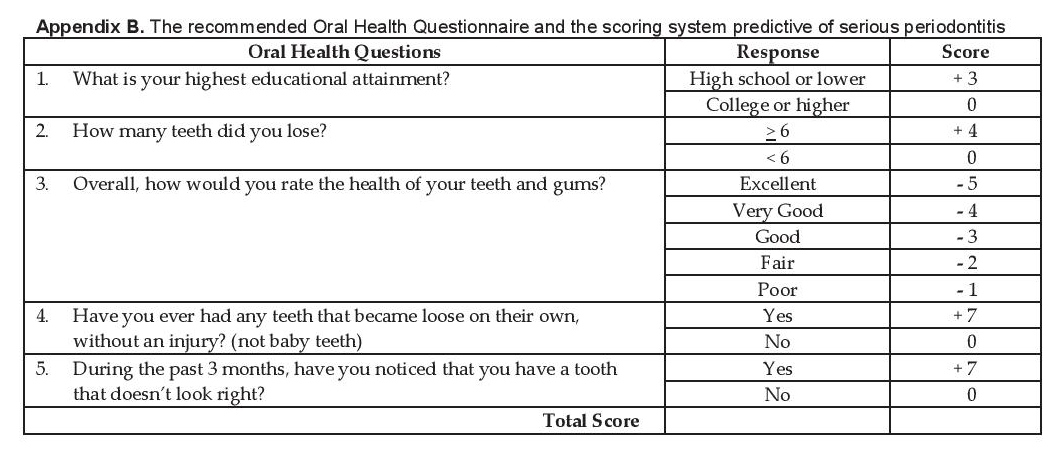
The Oral Health Screening Questionnaire for Persons with Diabetes (OHSQPD) was used in this study (Appendix A). The questionnaire is composed of 5 questions that pertain to (Q1) low education status, (Q2) tooth loss >6, (Q3) poor gum health, (Q4) presence of loose teeth and (Q5) poor tooth appearance and a scoring system designed to predict serious periodontitis (Appendix B). It is self-reported with all questions answerable by YES or NO answers.
Click here to download Appendix AAppendix A. The Oral Health Screening Questionnaire for persons with diabetes
Click here to download Appendix B
Appendix B. The recommended Oral Health Questionnaire and the scoring system predictive of serious periodontitis
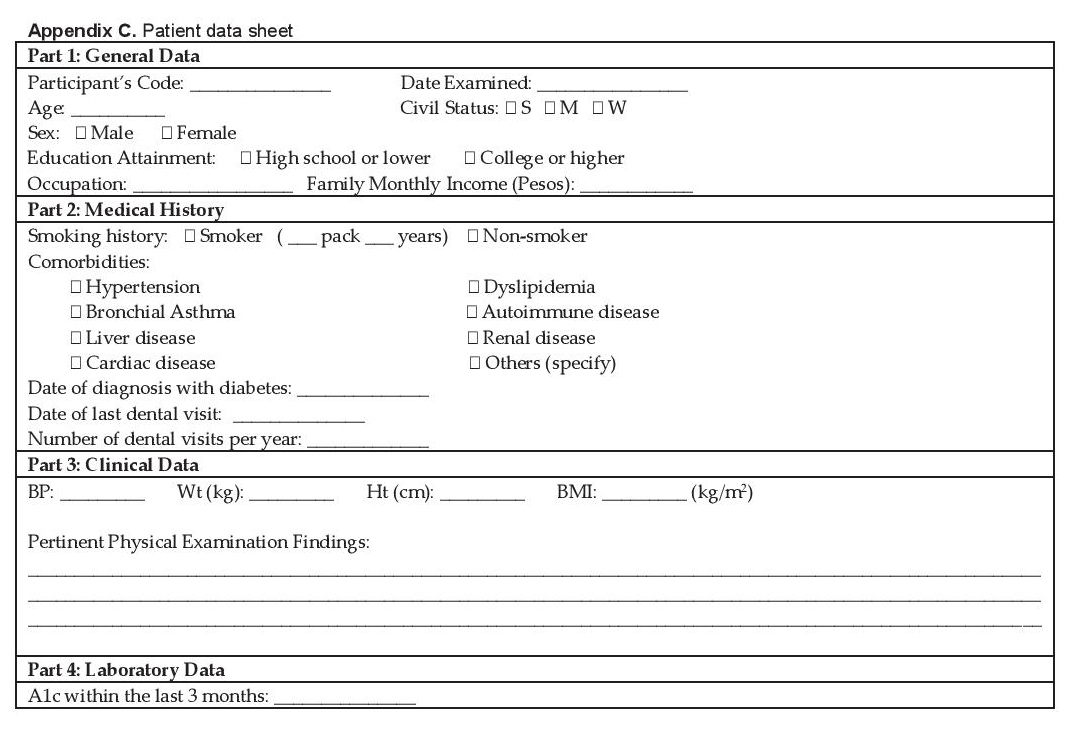
Participants included in the study were provided with an overview of the study and once eligibility status was determined, they were given the written informed consent and contact information was obtained. Information regarding gender, age, anthropometrics, smoking status, education level, duration of diabetes, frequency and last dental examination, co-morbidities, and A1c level were gathered. Socio-demographic and medical variables were collected using a standard data collection form (Appendix C).
Click here to download Appendix CAppendix C. Patient data sheet
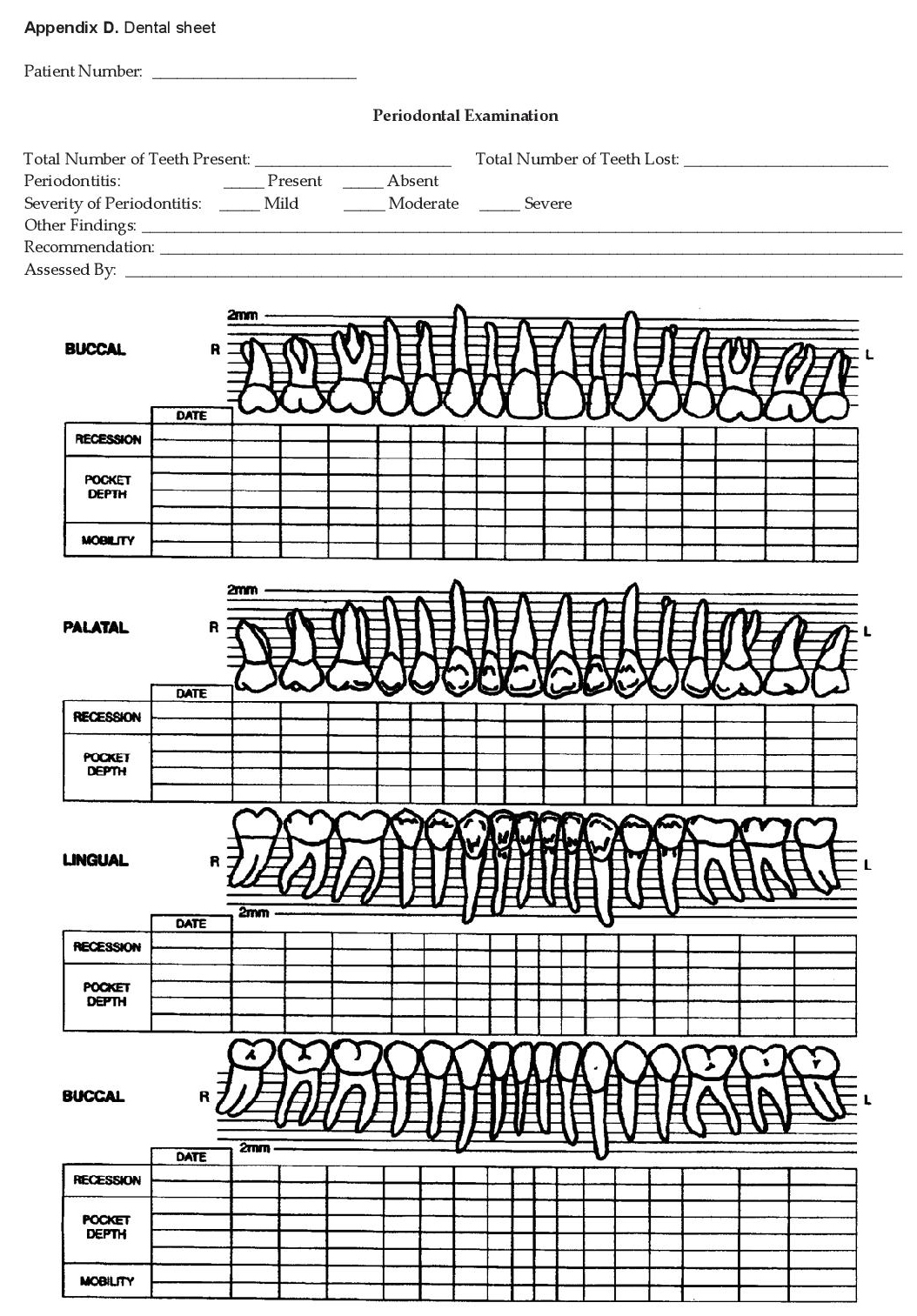
The participants answered the OHSQPD (Appendix A) and were then referred to a dentist (co-investigator), who was unaware of the answers for a formal dental and periodontal evaluation. The participants answered the OHSQPD (Appendix A) and were then referred to a dentist (co-investigator), who was unaware of the answers for a formal dental and periodontal evaluation. The basic elements from the NHANES III protocol were used for the full-mouth periodontal examination.[27][28] The dentist reported variables relating to the measurement of periodontal supporting tissues such as attachment loss, probing depth and furcation involvement. This was done using a color-banded probe graduated at 2, 4, 6, 8, 10, and 12 mm. Measurements were carried out on six sites per tooth (mesio-buccal, buccal, distobuccal, mesio-lingual, lingual, disto-lingual, mesiofacial, mid-facial, and distofacial) for all teeth (excluding 3rd molars). The number of lost teeth was also documented during the examination. Periodontal examination results were recorded using a separate Dental Sheet (Appendix D).
Click here to download Appendix DAppendix D. Dental sheet
Participants were classified according to the severity of periodontal disease based on the criteria used in the NHANES III.[28] Periodontitis was defined as a disease state in which there is an active destruction of the periodontal supporting tissues as evidenced by the presence of at least 3 mm probing depth and periodontal attachment loss at the same site. It is classified as follows:
- Severe periodontitis: 1) two or more teeth (or 30% or more of the teeth examined) having ≥5 mm probing depth, or 2) four or more teeth (or 60% or more of the teeth examined) having ≥4 mm probing depth, or 3) one or more posterior teeth with grade II furcation involvement.
- Moderate Periodontitis: 1) one or more teeth with ≥5 mm probing depth, or 2) two or more teeth (or 30% or more of the teeth examined) having ≥4 mm probing depth, or 3) one or more posterior teeth with grade I furcation involvement and accompanied by ≥3 mm probing depth.
- Mild periodontitis: 1) one or more teeth with ≥3 mm probing depth, or 2) one or more posterior teeth with grade I furcation involvement.
- No periodontitis: participants with 6 or more teeth present who did not fulfill any of the above criteria.
In this study, serious periodontal disease was considered for participants fulfilling the criteria for moderate to severe periodontitis.[11][29] Results of the periodontal evaluation were given to the participant. Intervention and follow-up were advised accordingly to ensure proper treatment of periodontitis.
Data AnalysisData analysis was done using the software Stata SE version 13. Quantitative variables were summarized as mean and standard deviation, while qualitative variables were tabulated as frequency and percentages. All responses on the oral health questionnaire were recorded according to the proposed scoring system (Appendix B).
The optimal cut-off value for detecting serious periodontitis was determined using a ROC. The value was determined using the point in which the sum of the sensitivity and specificity was highest.
The validity of the questionnaire in predicting serious periodontitis was assessed by determining its sensitivity, specificity, positive predictive value and negative predictive value (95% confidence interval) with the results of the full dental and periodontal examination as gold standard. The area under the receiver-operating curve (AUROC) (95% confidence interval) was computed to determine if the test is able to correctly classify those with and without the disease.
A total of 401 participants were consecutively seen in the UP-PGH outpatient clinics. One hundred seventy-seven (177) participants were not enrolled due to the exclusion criteria. The most common reason for exclusion was due to having fewer than 6 teeth left on examination. This accounted for 53% (93) of the excluded participants. Other leading reasons for exclusion were the 26% (47) who did not give consent and 16% (28) who had no recent A1c results. Figure 1 shows a flow diagram of the derivation of the participants available for the study.
Click here to download Figure 1Figure 1. Flow diagram of the derivation of participants.
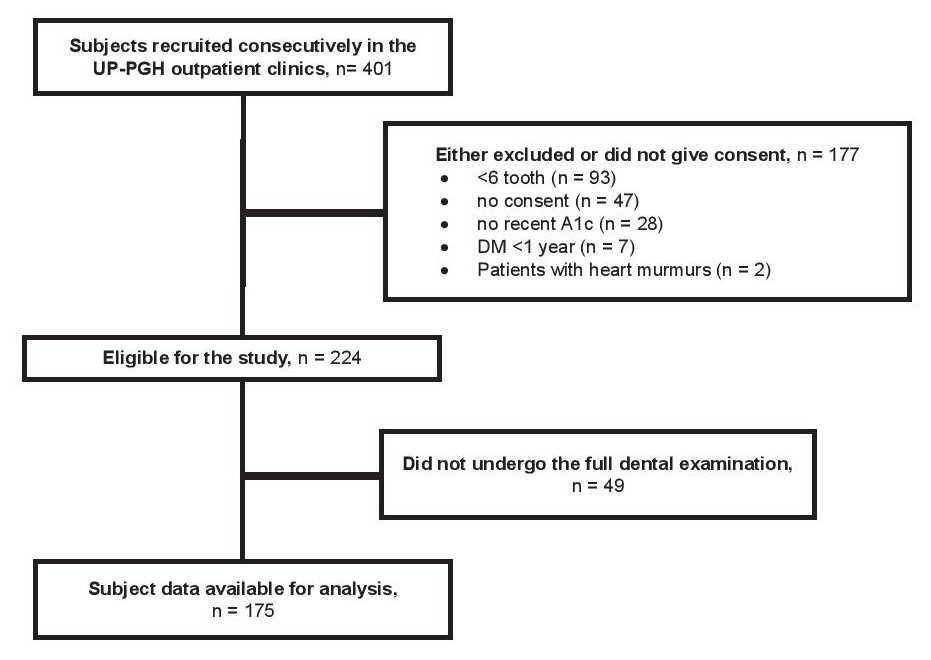
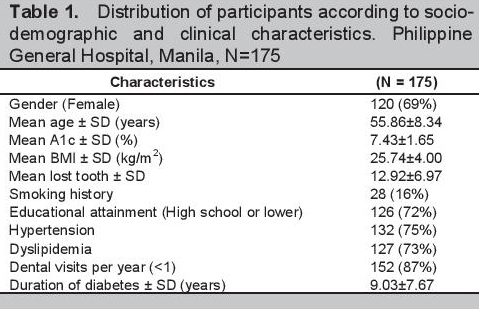
Of the 224 enrolled, 49 (22%) did not undergo the full dental examination so that the final data set for analysis included a total of 175 participants. The mean age of the participants was 55.9±8.3 years old (range 36-74) of which 120 (69%) were females. One hundred twenty-six (72%) did not reach or finish college. The mean BMI and A1c were 25.7±4.0 kg/m2 and 7.4±1.7% respectively with a mean duration of diabetes of 9.0±7.7 years. The majority of participants (87%) had no annual dental visits with a mean tooth loss on examination of 12.9±7.0. Only a minority of the participants were smokers or had ever smoked 28 (16%). Hypertension and dyslipidemia were the frequent co-morbidities observed. A summary of the distribution of the socio-demographic and clinical characteristics of the 175 participants included in the study is seen in Table 1.
Click here to download Table 1Table 1. Distribution of participants according to sociodemographic and clinical characteristics. Philippine General Hospital, Manila, N=175
Overall, 93% (162) of the participants had periodontitis, while the prevalence of serious periodontitis (moderate and severe) was 61% (107). The prevalence of mild, moderate, and severe periodontitis were 7.5% (13), 31% (55) and 54% (94) respectively. Only 7.5% (13) had no periodontitis on examination.
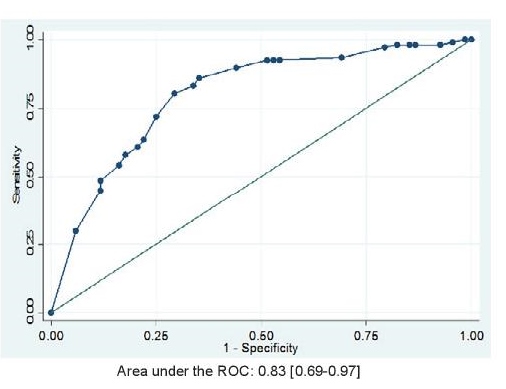
The optimal cut-off value for detecting serious periodontitis based on study criteria determined using the ROC was 12 (see Figure 2). With this cut-off score, the estimated prevalence of serious periodontitis was also 61% (106). The distribution of participants according to the oral health scores, seriousness of periodontitis, and the validity characteristics of the self-report questionnaire are seen in Tables 2 and 3. The questionnaire yielded a sensitivity [95% CI] of 80.4% [72.9-87.9] and a specificity [95% CI] of 70.6% [59.8-81.4]. Positive and negative predictive values were 81.0% [72.6-89.1] and 70% [58.7-80.4] respectively. The area under the receiver operating curve (AUROC) [95% CI] was 0.83 [0.69-0.97] (Figure 2).
Click here to download Table 2Table 2.Distribution of the participants according to the oral health score and seriousness of periodontitis
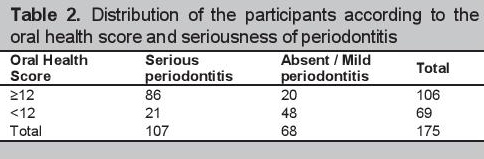
Click here to download Table 3
Table 3.Validity characteristics of the Oral Health Screening Questionnaire for Diabetics at a derived cut-off score of 12
Click here to download Figure 2
Figure 2. Area under the Receiver Operating Curve (AUROC) for identifying serious periodontitis using the self-reported Oral Health Questionnaire, N =175.
Periodontitis is a complication of diabetes and it causes a significant burden. There is still a lack of a certain degree of awareness regarding oral health and its relationship with diabetes. It is clear that routine oral health care in adults with diabetes is uncommon, as 152 (87%) of the participants had no regular dental visits.
While a full dental and periodontal examination remains the standard of care for persons with diabetes, it comes at a cost. Oral health is an important element of diabetes care and will contribute to the improvement in glycemic control.15 Self-report measures can offer a practical alternative for periodontal disease evaluation. The OHSQPD was inexpensive and easy to administer in the outpatient setting. The importance of validating the questionnaire then, is to identify persons with diabetes having serious periodontitis who will potentially require urgent dental evaluation and treatment.
One hundred sixty two (93%) participants had periodontitis while more than half (107 or 61%) of the population had serious periodontitis. This prevalence of periodontitis based on the full dental and periodontal examination is similar to what was reported by Lo et. al. and this may be due to the similar population characteristics such as a lower level of education and poorer oral health care.[11]
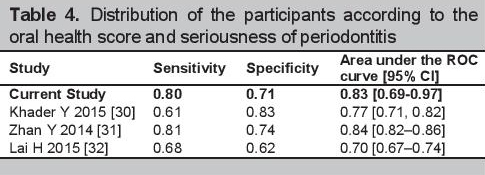
Currently, there are no studies that used self-report questionnaires to detect periodontitis among persons with diabetes, however when compared to other self-reported periodontal disease scales that predicted periodontitis in those without diabetes, the questionnaire had comparable sensitivity and specificity.[30]-[32] The validity characteristics of these self-reported periodontal disease scales for detecting serious periodontitis are seen in Table 4.
Click here to download Table 4Table 4. Distribution of the participants according to the oral health score and seriousness of periodontitis
As the study is done in a tertiary referral center, the questionnaire might perform differently in the community setting. Limitations stem from the setting of the study and are due to the educational and language barriers that may be encountered. Validity therefore may be dependent on the specific population characteristics. These population characteristics may affect the comprehensibility of the self-report questions hence may influence participant responses. The participants included in this study are also relatively older and already with established diabetes for almost ten years; thus, the sensitivity and specificity of the questionnaire in detecting serious periodontitis may be different in younger populations with a shorter duration of diabetes. Further evaluation is needed to determine the performance of the questionnaire in the community setting.
The Oral Health Screening Questionnaire for Persons with Diabetes is a valid tool with good sensitivity, specificity and predictive value for detecting serious periodontitis. In can potentially become an invaluable tool in settings in which routine and clinical oral examination for all diabetics is not feasible.
Statement of AuthorshipAll authors have given approval to the final version submitted.
Author DisclosureAll the authors have declared no conflict of interest to the work carried out in this paper.
Funding SourceNone.
[1] International Diabetes Federation. IDF Atlas, seventh edition, 2015. Available from: http://www.diabetesatlas.org/.
[2] FNRI-DOST. Burden of Selected Risk Factors to Non-Communicable Diseases among Filipino Adults. The 8th National Nutrition Survey, 2013. Retrieved from http://obesity.org.ph/v4/wpcontent/uploads/2013/04/8thNNSResultsNCD.pdf.
[3] Yuen HK, Onicescu G, Hill EG, Jenkins C. A survey of oral health education provided by certified diabetes educators. Diabetes Res Clin Pract. 2010; 88(1):48-55. DOI.
[4] Löe H. Periodontal disease: The sixth complication of diabetes mellitus. Diabetes Care. 1993;16(1):329–34. DOI.
[5] Wu YY, Xiao E, Graves DT. Diabetes mellitus related bone metabolism and periodontal disease. Int J Oral Sci. 2015;26(7):63-72.
[6] Schmidt AM, Weidman E, Lalla E, et al. Advanced glycation endproducts (AGEs) induce oxidant stress in the gingiva: A potential mechanism underlying accelerated periodontal disease associated with diabetes. J Periodontal Res.1996;31(7):508–15. DOI.
[7] Preshaw PM, Bissett SM. Periodontitis: Oral complication of diabetes. Endocrinol Metab Clin North Am. 2013;42(4): 849–67. DOI.
[8] Apoorva SM, Sridhar N, Suchetha A. Prevalence and severity of periodontal disease in type 2 diabetes mellitus (non-insulin-dependent diabetes mellitus) patients in Bangalore city: An epidemiological study. J Indian Soc Periodontol. 2013;17(1):25-9. DOI.
[9] Kowall B, Holtfreter B, Völzke H, et al. Pre-diabetes and well-controlled diabetes are not associated with periodontal disease: The SHIP trend study. J Clin Periodontol. 2015;42(5):422-30. DOI.
[10] Bitong ED, Jasul GV, Dellosa MAG. Prevalence of periodontitis and its association with glycemic control among patients with type 2 diabetes mellitus seen at St. Luke’s Medical Center. Philipp J Intern Med. 2010; 48(1):9-14.
[11] Lo TE, Lagaya-Estrada MC, Jimeno C, Jasul G. Clinical utility of selfreported oral health measures for predicting periodontitis among adult Filipinos with type 2 diabetes mellitus. J ASEAN Fed Endocr Soc. 2016;31(1):10-17. DOI.
[12] Jimeno CA. Updates on the UNITE for Diabetes Philippine Practice Clinical Practice Guidelines for Diabetes Part 2. PPD Compendium of Philippine Medicine, 2014.
[13] Kiran M, Arpak N, Unsal E, Erdoğan MF. The effect of improved periodontal health on metabolic control in type 2 diabetes mellitus. J Clin Periodontol. 2005;32(3):266–72. DOI.
[14] Engebretson S, Kocher T. Evidence that periodontal treatment improves diabetes outcomes: A systematic review and meta-analysis. J Clin Periodontol. 2013;40(Suppl 14):S153–69.
[15] Teeuw WJ, Gerdes VEA, Loos BG. Effect of periodontal treatment on glycemic control of diabetic patients. Diabetes Care. 2010;33(2):421-7. DOI.
[16] Ravindran R, Deepa MG, Sruthi AK, et al. Evaluation of oral health in type 2 diabetes mellitus patients. Oral Maxillofac Pathol J. 2015;6(1):525-31. DOI.
[17] Ofilada EJ, Jimeno C. A survey on the barriers to dental care among individuals with type 1 diabetes mellitus. Philipp J Intern Med. 2013;51(2):1-6.
[18] Chaudhari M, Hubbard R, Reid RJ, et al. Evaluating components of dental care utilization among adults with diabetes and matched controls via hurdle models. BMC Oral Health. 2012;12:20. DOI.
[19] Becker W, Berg L, Becker BE. Untreated periodontal disease: A longitudinal study. J Periodontol.1979;50(5):234-44. DOI.
[20] Matuliene G, Pjetursson BE, Salvi GE, et al. Influence of residual pockets on progression of periodontitis and tooth loss: Results after 11 years of maintenance. J Clin Periodontol. 2008;35(8):685-95. DOI.
[21] Lorrentz TCM, Cota LOM, Cortelli JR, Vargas AMD, Costa FO. Tooth loss in individuals under periodontal maintenance therapy: Prospective study. Braz Oral Res. 2010;24(2):231-7. DOI.
[22] Mealey BL, Ocampo GL. Diabetes mellitus and periodontal disease. Periodontol 2000. 2007;44(1):127–53. DOI.
[23] Eke PI, Genco RJ. CDC periodontal disease surveillance project: Background, objective, and progress report. J Periodontol. 2007;78(7s):1366-71. DOI.
[24] Eke PI and Dye B. Assessment of self-report measures for predicting population prevalence of periodontitis. J Periodontol. 2009;80(9):1371- 9. DOI.
[25] Miller K, Eke PI, Schoua-Glusberg A. Cognitive evaluation of selfreport questions for surveillance of periodontitis. J Periodontol. 2007;78(7s):1455-62. DOI.
[26] American Diabetes Association. Standards of Medical Care in Diabetes. Diabetes Care. 2015;38(Suppl 1):S1-93.
[27] Dye BA, Barker LK, Selwitz RH, et al. Overview and quality assurance for the National Health and Nutrition Examination survey (NHANES) oral health component, 1999-2002. Community Dent Oral Epidemiol. 2007;35(2):140-51. DOI.
[28] Albandar JM, Brunelle JA, Kingman A. Destructive periodontal disease in adults 30 years of age and older in the United States, 1988- 1994. J Periodontol. 1999;70(1):13-29. DOI.
[29] Beck JD, Koch GG, Rozier RG, Tudor GE. Prevalence and risk indicators for periodontal attachment loss in a population of older community-dwelling blacks and whites. J Periodontol. 1990;61(8):521- 8. DOI.
[30] Khader Y, Alhabashneh R, Alhersh F. Development and validation of a self-reported periodontal disease measure among Jordanians. Int Dent J. 2015;65(4):203-10. DOI.
[31] Zhan Y, Holtfreter B, Meisel P, et al. Prediction of periodontal disease: Modelling and validation in different general German populations. J Clin Periodontol. 2014;41(3):224–31. DOI.
[32] Lai H, Su CW, Chiu SY, et al. A prediction model for periodontal disease: Modelling and validation from a National Survey of 4061 Taiwanese adults. J Clin Periodontol. 2015;42(5):413–21. DOI.
Authors are required to accomplish, sign and submit scanned copies of the JAFES Author Form consisting of: (1) the Authorship Certification that the manuscript has been read and approved by all authors, and that the requirements for authorship have been met by each author, (2) the Author Declaration that the article represents original material that is not being considered for publication or has not been published or accepted for publication elsewhere, (3) the Statement of Copyright Transfer[accepted manuscripts become the permanent property of the JAFES and are licensed with an Attribution-Share Alike-Non-Commercial Creative Commons License. Articles may be shared and adapted for non-commercial purposes as long as they are properly cited], (4) the Statement of Disclosure that there are no financial or other relationships that might lead to a conflict of interest. For Original Articles involving human participants, authors are required to submit a scanned copy of the Ethics Review Approval of their research. For manuscripts reporting data from studies involving animals, authors are required to submit a scanned copy of the Institutional Animal Care and Use Committee approval. For Case Reports or Series, and Images in Endocrinology, consent forms, are required for the publication of information about patients. Articles and any other material published in the JAFES represent the work of the author(s) and should not be construed to reflect the opinions of the Editors or the Publisher.