The incidence of thyroid cancer has continuously increased in the last three decades all over the world.[1] In the Philippines, the incidence has been stable, with an annual average increase of 0.4% and 1.6% in males and females, respectively, as of 2002.[2] High survival rates (five-year survival rates around 98.1% from 2006-2012 in the US) account for the growing number of thyroid cancer survivors.[3]
The treatment of thyroid cancer is particularly effective in early stage disease and involves surgery (total thyroidectomy or lobectomy with or without lymphadenectomy), which is usually followed by radioiodine ablation therapy and suppressive doses of levothyroxine. These treatment modalities, however can be associated with physical or psychological complaints as shown in studies by Almeida et al.,[4] Rubic et al., [5] and Gomez et al.,[6] which revealed radioactive iodine-related complaints like problems in swallowing, hypothyroid symptoms such as fatigue and negative effects on psychological well-being in patients with thyroid cancer. Moreover, the disease can recur even after several decades or persist for years requiring treatment. Therefore, long term follow-up is needed which can also lead to psychological distress. All of these factors could significantly alter the health-related quality of life (HRQoL) of thyroid cancer patients.
HRQoL is defined by the World Health Organization as a subjective perception of health in terms of physical, mental, and social well-being of a patient.[7] Assessment of HRQoL in thyroid cancer patients can reveal the significant concerns of the patients relating to the disease. There are several studies assessing the HRQoL of thyroid cancer patients. Husson et al., systematically reviewed the literature on HRQoL of thyroid cancer survivors yielding 27 studies. The review showed that thyroid cancer survivors generally have a similar or slightly worse HRQoL compared with the normative population. Some of the identified causes in decrease in HRQoL include mental problems like anxiety and depression, physical problems like hoarseness, fatigue, chills and tingling sensation and decrease in social functioning in thyroid cancer patients in comparison to normal people in some of the included studies.[8] The studies, however, used general HRQoL questionnaires, which might not reveal specific thyroid cancer-related complaints or a non-validated thyroid cancer HRQoL questionnaire.
The European Organization for Research and Treatment of Cancer (EORTC) created a quality of life questionnaire (EORTC QLQ-C30) which is one of the most widely used questionnaires for assessing quality of life in patients with cancer. This was meant to be used with supplementary modules that evaluate HRQoL in specific diseases.[9] No module currently exists specifically for thyroid cancer. The only validated thyroid cancer-specific HRQoL (THYCA-QoL) questionnaire was developed in a Dutch population of thyroid cancer patients by Husson et al., according to the methods of the EORTC Quality of Life group.[10] Currently, there is no validated thyroid cancerspecific HRQoL questionnaire in the Philippines.
A HRQoL questionnaire specific for thyroid cancer patients that can be used together with the EORTC QLQC30 in the Philippines will be very helpful in assessing the areas in these patients’ lives that need to be addressed by physicians to maintain and/or improve quality of life. Differences between populations in terms of culture, socioeconomic status and probably treatment practices in thyroid cancer are some of the reasons for developing a new questionnaire instead of just adapting a validated questionnaire from another country.
The aim of the study was to develop a thyroid cancerspecific quality of life questionnaire in combination with the core cancer quality of life questionnaire EORTC QLQC30 and validate its use for adult Filipinos with differentiated thyroid cancer.
METHODOLOGYStudy Designs
The first part of the research (Phases I-III) involved questionnaire development. The second part (Phase IV) was a cross sectional analytic study to validate the questionnaire developed in the previous phases.
Study SubjectsThe study subjects were adult patients aged 19 years and above with well differentiated thyroid cancer (DTC) who were recruited from the Philippine General Hospital, a tertiary hospital in Manila. The diagnosis of DTC was based on histopathology results after thyroidectomy with or without maintenance levothyroxine therapy. Subjects were only included after giving their informed consent to participate in this study.
Subjects were excluded if they satisfied any of the following criteria: presence of cognitive impairment; presence of severe/uncontrolled comorbid diseases (uncontrolled hypertension, uncontrolled diabetes mellitus, congestive heart failure, chronic obstructive pulmonary disease, cerebrovascular disease, myocardial infarction, chronic kidney disease); presence of another coexistent cancer; and being illiterate or unable to read and write.
The study protocol was submitted to the University of the Philippines Manila Research Ethics Board (UPMREB) Panel for ethics review and approval. Implementation of the study began after approval from UPMREB. All patient information was kept anonymous and confidential. There was no conflict of interest in this study.
Description of ProcedureThe development of a thyroid cancer-specific HRQoL questionnaire was partially based on the EORTC Quality of Life Group Guidelines for Developing Questionnaire Modules.[11]
Phase I: Preliminary Steps - Generation of Health- Related Quality of Life Components (Review of Literature, Focus Group Discussions with patients and expert panel)Phase I was intended to generate a comprehensive list of relevant HRQoL issues for thyroid cancer patients. Literature searches were done in PUBMED and Google Scholar to identify all relevant HRQoL issues. Existing, relevant questionnaires were also reviewed. A list of all questionnaires identified and finally a list of all potentially relevant HRQoL issues were then created.
A focus group of subjects with differentiated thyroid cancer [with representatives from the different types of differentiated thyroid cancer (i.e., papillary and follicular thyroid cancer), from the different stages of the cancer, from age groups <45 and 45 or greater, and from patients <10 years and 10 or more years from diagnosis] was formed in order to discuss relevant HRQoL issues related to their disease. The researcher asked the patients to describe their experiences and showed them existing HRQoL issues from the literature review during the discussion to determine their opinions regarding them. The interviews continued until no new issue was raised. Issues raised during the discussion that were not found in the initial list were added.
The provisional list of issues was then evaluated by an expert panel of health care professionals to assess aptness of content and broadness of coverage. Five health professionals (2 endocrinologists, 1 otorhinolaryngologisthead and neck surgeon, 1 nuclear medicine specialist, 1 general surgeon with specialty in head and neck surgery) were included in the panel. The specialists were asked to identify the issues that, in their opinion, affect patients' HRQoL most profoundly and check if there were any missing HRQoL complaints. Additional issues based on the discussion were added to the list.
The health care providers and a sample of thyroid cancer patients [with representatives from the different types of differentiated thyroid cancer (i.e., papillary and follicular thyroid cancer), from the different stages of the cancer, from age groups <45 and 45 or greater, and from patients <10 years and 10 or more years from diagnosis] not involved in the focus group discussion were then asked to rate the issues from 1 (not relevant for thyroid cancer patients) to 4 (very relevant for thyroid cancer patients) on a Likert scale (relevance rating) and to select at the most 25 issues which they thought must be included in the questionnaire (priority rating).
Issues with high relevance ratings (mean score ≥1.5) and high priority ratings for inclusion in the module (ratings ≥25%), based on the recommendations by EORTC[11]
and the study by Husson et al.,[10] for both health care providers and thyroid cancer patients were included in the final list of issues. Issues that were already present in the EORTC QLQ-C30 questionnaire, and those that were upsetting to patients were excluded. Phase II: Construction of the Draft QuestionnaireThe final list of HRQoL issues from Phase I was then structured into questions similar in format with the EORTC QLQ-C30 (response categories: ‘not at all,’ ‘a little,’ ‘quite a bit’ and ‘very much’). Issues which had been formed into question items in previous EORTC modules were used for uniformity of the questionnaire using the EORTC QoL Item Bank with permission from the authors. For the items that are unavailable in the EORTC Item Bank, new questions were constructed. The questions were created as clear, brief and unambiguous as possible.
The resulting provisional list of items were reviewed for clarity and overlap by the panel of health care professionals and a social scientist, after which the prefinal questionnaire was translated into Filipino for use in the pre-testing phase.
The translation was conducted by two translators who were native speakers of Filipino who have high level of fluency in English. They independently translated the questionnaire into Filipino. The first and the second translators’ versions were merged into one single forward translation by the primary investigator. Then two translators translated the questionnaire from the provisional forward translation back into English. The two English back-translation versions were checked by the primary investigator for consistency.
Phase III: Pilot Testing of the Draft QuestionnaireThe final list of questions after Phase II was then pretested in a small number of patients with differentiated thyroid cancer [with representatives from the different types of differentiated thyroid cancer (i.e., papillary and follicular thyroid cancer), from the different stages of the cancer, from age groups <45 and 45 or greater, and from patients <10 years and 10 or more years from diagnosis] who were not involved in Phase I, after completing the EORTC QLQC30, to determine problems relating to the construction and comprehensibility of items. Interviews were conducted with the patients after completion of the questionnaire to ensure completeness and acceptability of the items in the list. The time it took study subjects to answer the questionnaire was recorded.
At this stage in Phase 3, a selection process which was determined beforehand was applied to remove unnecessary items. The following cut-off points were used for selection of items for retention in the final module: mean Likert scale score >1.5, prevalence ratio >30%, range >2 points, no floor or ceiling effect: responses in categories 3 and 4 or 1 and 2 >10%, no significant concerns expressed by patients (e.g. item is upsetting, ambiguous), compliance of at least 95% response to the item. Items that met five of the six criteria mentioned above were retained in the list while those that did not were excluded.
The final list of items based on the above criteria comprised the pre-final questionnaire.
Phase IV: Validation of the Pre-final QuestionnaireThe resultant pre-final questionnaire was validated in a sample of well differentiated thyroid cancer patients (at least 10 patients per question item based on the recommendation by Nunnally[12]). These subjects included a diverse group of DTC patients [with representatives from the different types of differentiated thyroid cancer (i.e., papillary and follicular thyroid cancer), from the different stages of the cancer, from age groups <45 and 45 or greater, and from patients <10 years and 10 or more years from diagnosis], not involved in the previous phases of the study, from both charity and pay patients who consulted at the outpatient clinic or were admitted at the Philippine General Hospital. The subjects first completed the EORTC QLQ-C30 before answering the pre-final version of the questionnaire.
Data analyses included assessment of the response distributions for each item to examine central tendency and variability, and determine presence or absence of ceiling and floor effects; evaluation of construct validity using factor analysis; determination of scale structure using multi-trait scaling analysis; assessment of reliability using Cronbach’s alpha coefficient; and ascertainment of correlation of the developed questionnaire with the EORTC QLQ-C30 utilizing Spearman correlation. Floor or ceiling effect (when 80% of the responses fall in one response category) impairs the ability of investigators to determine the central tendency of the data, thus question items containing either of these were removed from the questionnaire.
Phase I
The literature search using the keywords: "quality of life," "health-related quality of life," and "complaints" in addition to "thyroid cancer" or "thyroid carcinoma" yielded 47 studies and two thyroid cancer specific questionnaires (1 validated in a Dutch population and another non-validated questionnaire). A total of 81 HRQoL issues were identified from the studies and questionnaires.
The focus group discussion involving 6 diverse thyroid cancer patients yielded 3 more HRQoL issues. Another 9 issues were added and 7 were removed based on discussion with the expert panel. Twenty-four issues were removed because they were already covered by the EORTC QLQ-C30. A total of 62 HRQoL issues remained. These were presented to the 5 health care professionals and 20 diverse thyroid cancer patients for relevance and priority ratings using an English and Filipino Likert scale rating and priority rating tables, respectively. Thirty-four issues were removed because of low relevance and low priority ratings. A total of 28 HRQoL issues relevant to thyroid cancer patients were retained (Appendix A).
Click here to download Appendix AAppendix A. Final list of HRQoL issues from Phase I of study

Phase II
Four HRQoL issues were constructed into items using questions from the EORTC QoL Item Bank and the remaining 24 issues which were not found in the item bank, were constructed into new questions. These questions were then reviewed by the expert panel and a social scientist resulting in a pre-final list of questions (Appendix B). The time period for the health related complaints assessed by the EORTC QLQ-C30 questionnaire is one week. However, after discussion with the panel and thyroid cancer patients, it was decided to use one month for this study because it was felt that one week is too short for the HRQoL issues in patients with thyroid cancer.
Click here to download Appendix BAppendix B. List of constructed questions

Translation of the constructed questions from English to Filipino and then back to English was done. The Filipino forward translated version had good correlation with the initially formed English questionnaire.
Phase IIIPretesting was done on 15 thyroid cancer patients. Patients did not find any annoying, confusing, upsetting, intrusive or irrelevant questions. They also did not think there were missing items from the list. There were no identified problems with the phrasing of the questions. Patients finished the questionnaire with an average time of 6 minutes, with a range of 3 to 9 minutes.
After Phase III of the study, 6 items were removed because of failure to meet 5 of 6 criteria for retention. The recommended number of items at the end of this phase is about 20. In this study there were 22.
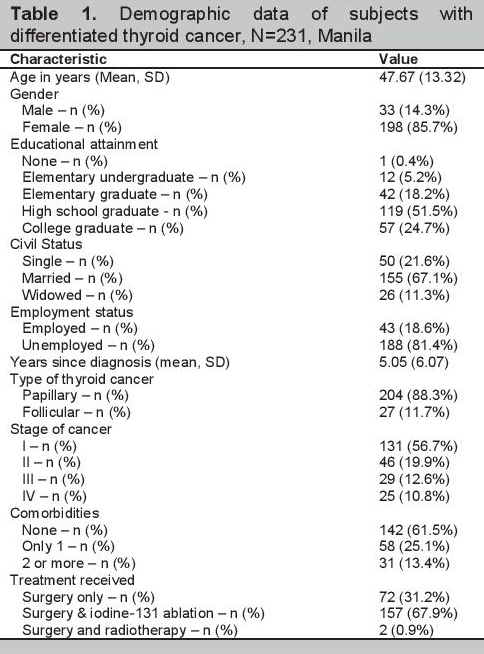
Phase IVValidation was done in 231 patients with DTC. Table 1 shows the demographic data of the subjects. Of the 231 participants, 33 (14%) were men and 198 (86%) were women. The mean age of participants was 47.67±13.32 years (range 19-79 years). Fifty (22%) were single, 155 (67%) were married, and 26 (11%) were widowed. They possessed varying degrees of formal education, with one having no formal education at all, 12 (5%) were elementary undergraduates, 42 (18%) were elementary graduates, 119 (52%) were high school graduates, and 57 (25%) were college graduates. Eighty-one percent was unemployed. Time since diagnosis of thyroid cancer ranged from 29 days to 42 years, with a mean time of 5.05±6.07 years. Two hundred four (88%) had papillary type of thyroid cancer and 27 (12%) had follicular type. The participants had different stages of cancer: 131 (57%) were stage 1, 46 (20%) were stage 2, 29 (13%) were stage 3, and 25 (11%) were stage 4. While 61% had no comorbidity, 25% had one and 13% had two comorbidities. All participants underwent surgery. Of these, two (0.87%) also had radiotherapy and 157 (68%) had iodine-131 ablation.
Click here to download Table 1Table 1. Demographic data of subjects with differentiated thyroid cancer, N=231, Manila
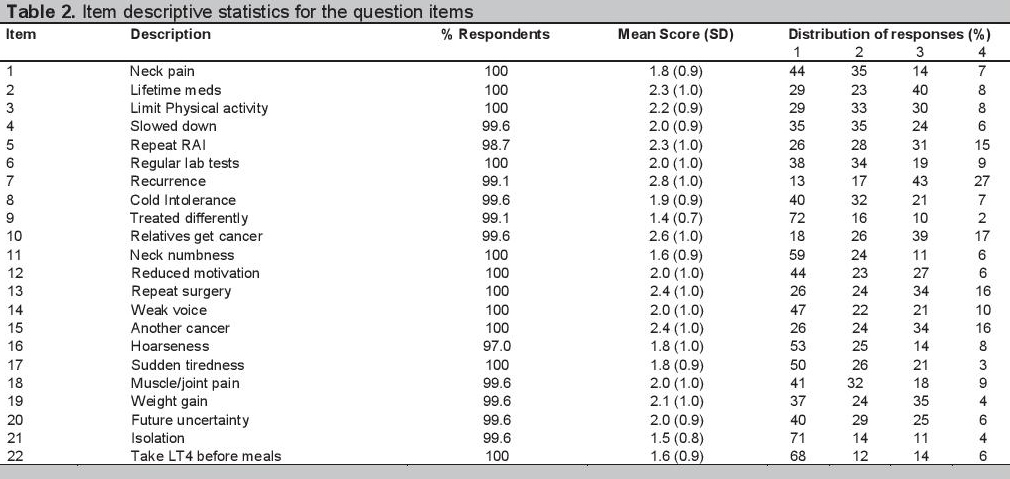
Data analyses were done after completion of Phase IV to determine which items to retain and which to exclude. Item descriptive statistics (Table 2) showed high valid responses and absence of floor or ceiling effects for all items. Thus, no item was excluded at this point in the study.
Click here to download Table 2Table 2. Item descriptive statistics for the question items
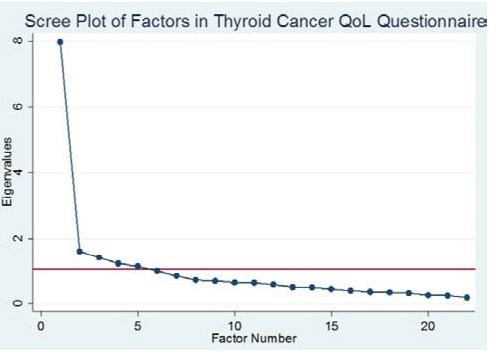
To assess construct validity, factor analysis was done. The suitability of the data for factor analysis was tested via the Kaiser-Meyer-Olkin (KMO) measure of sampling adequacy. For a satisfactory analysis to proceed, the KMO value should be higher than 0.5. The KMO measure in the study was 0.89, thus factor analysis was done. Five factors were identified based on Cattell’s scree plot which states that an eigenvalue (the amount of the total variance explained by that factor) must be greater than one for a factor to be retained. Figure 1 shows that five factors have eigenvalues of at least 1 and were thus retained.
Click here to download Figure 1Figure 1. Scree Plot shows that five factors have eigenvalues of at least 1 which means that these five factors explain most of the variability in the data.
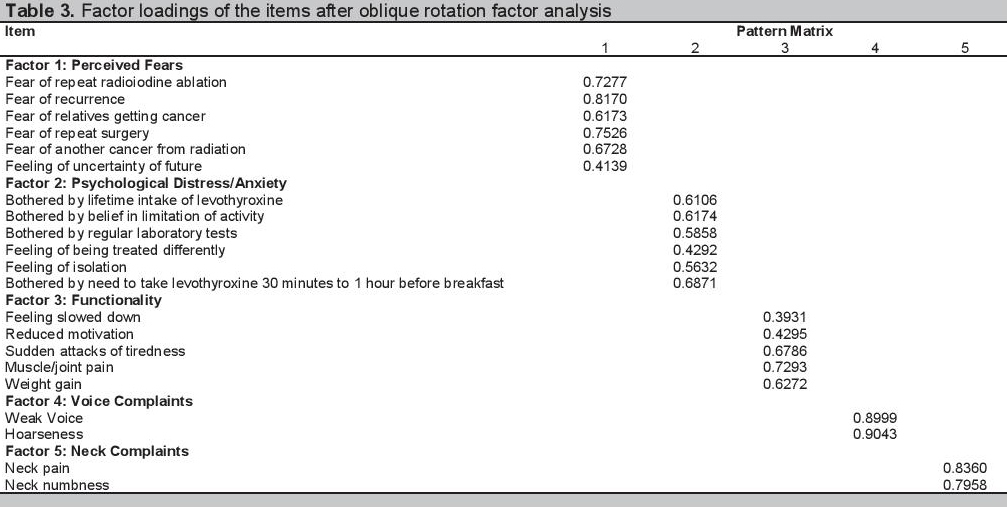
Oblique rotation to determine factor loadings of each item was done (Table 3). The items that had high factor loadings (>0.35) in the same factor were grouped together indicating that these items may reflect a related groups of symptoms or complaints. Factor 1 is defined by items relating to perceived fears of the patients. Factor 2 is composed of items relating to psychological distress or anxiety. Factor 3 includes items relating to functionality of patients. Factors 4 and 5 are items dealing with voice complaints and neck complaints, respectively. Some items had high item loadings in more than 1 factor but were assigned to the factor with the items they are more compatible with. The item for cold intolerance did not have high loading in any of the factors and thus was considered as a single item. Click here to download Table 3
Table 3. Factor loadings of the items after oblique rotation factor analysis
The reliability of the five identified factors or scales was assessed using Cronbach’s alpha. Acceptable Cronbach’s alpha is 0.7. The convergent and divergent validity were assessed using Spearman correlation. A value of >0.4 indicate moderate to high correlation while <0.4 indicate weak correlation. Items of the same scale should have moderate to high correlation or good convergent validity while items from different scales should have weak correlation or good divergent validity.
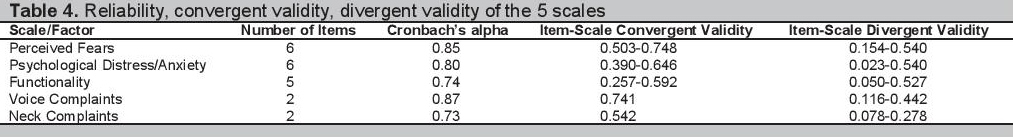
Cronbach’s alpha reliability coefficients for the five factors range from 0.73-0.87. All are greater than the preferred cut-off of 0.7 which indicates good reliability. The overall Cronbach’s alpha for the entire measure is 0.91. All the scales in general have good convergent and divergent validity with some exceptions (Table 4).
Click here to download Table 4Table 4. Reliability, convergent validity, divergent validity of the 5 scales
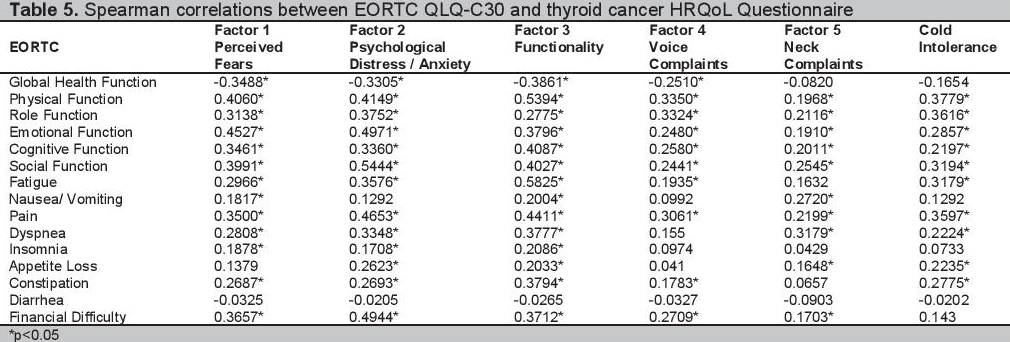
Furthermore, Spearman correlations between the various scales of the EORTC QLQ-C30 and the scales and items of the created thyroid cancer specific questionnaire were done. Most scales of the thyroid cancer HRQoL questionnaire correlated poorly with the EORTC QLQC30 (Table 5).
Click here to download Table 5Table 5. Spearman correlations between EORTC QLQ-C30 and thyroid cancer HRQoL Questionnaire
This questionnaire was developed using the guidelines of EORTC, which is one of the largest questionnaire-making body for quality of life of cancer patients. Extensive literature search, and thorough interviews and consult with relevant health care professionals and thyroid cancer survivors were done to create a questionnaire that covers the multidimensional HRQoL issues in patients with DTC.
There were 5 factors or scales identified in this study. The scales all have good reliability and mostly good convergent validity (correlation of >0.4) with some exceptions in Factors 2 and 3 and good divergent validity (correlation of <0.4) with some exceptions in the 5 factors which may be explained by loading of some items in more than 1 factor. Thus, all the items were retained. Spearman correlation between the EORTC QLQ-C30 and the developed thyroid cancer questionnaire was weak which indicates that there is no redundancy between the two questionnaires. Thus, the developed and validated thyroid cancer HRQoL questionnaire can be used in combination with the EORTC QLQ-C30 for patients with DTC.
There are a few existing questionnaires specifically used in the assessment of HRQoL of thyroid cancer patients. The THYCA-QoL is a thyroid cancer HRQoL questionnaire developed and validated for the Dutch population.[10]
Jeong et al., also validated a Korean version of this questionnaire which was shown to be a reliable and valid assessment tool that can be used in combination with the EORTC QLQ-C30 to assess the HRQoL of Korean thyroid cancer patients.[13] Most of the items in that questionnaire are related to physical complaints. In contrast, the results of this study showed several psychological as well as functional HRQoL complaints in addition to physical complaints in Filipino patients. This study shows that differences in complaints or perception of a decrease in quality of life occur in different cultures of subjects with DTC.Another thyroid cancer HRQoL questionnaire known as City of Hope Quality of Life – Thyroid version was developed in California, USA.[14] However, it was not validated. It includes physical, psychological, social and spiritual components. EORTC is also currently developing a thyroid cancer specific HRQoL questionnaire which will be validated in the European population.[15]
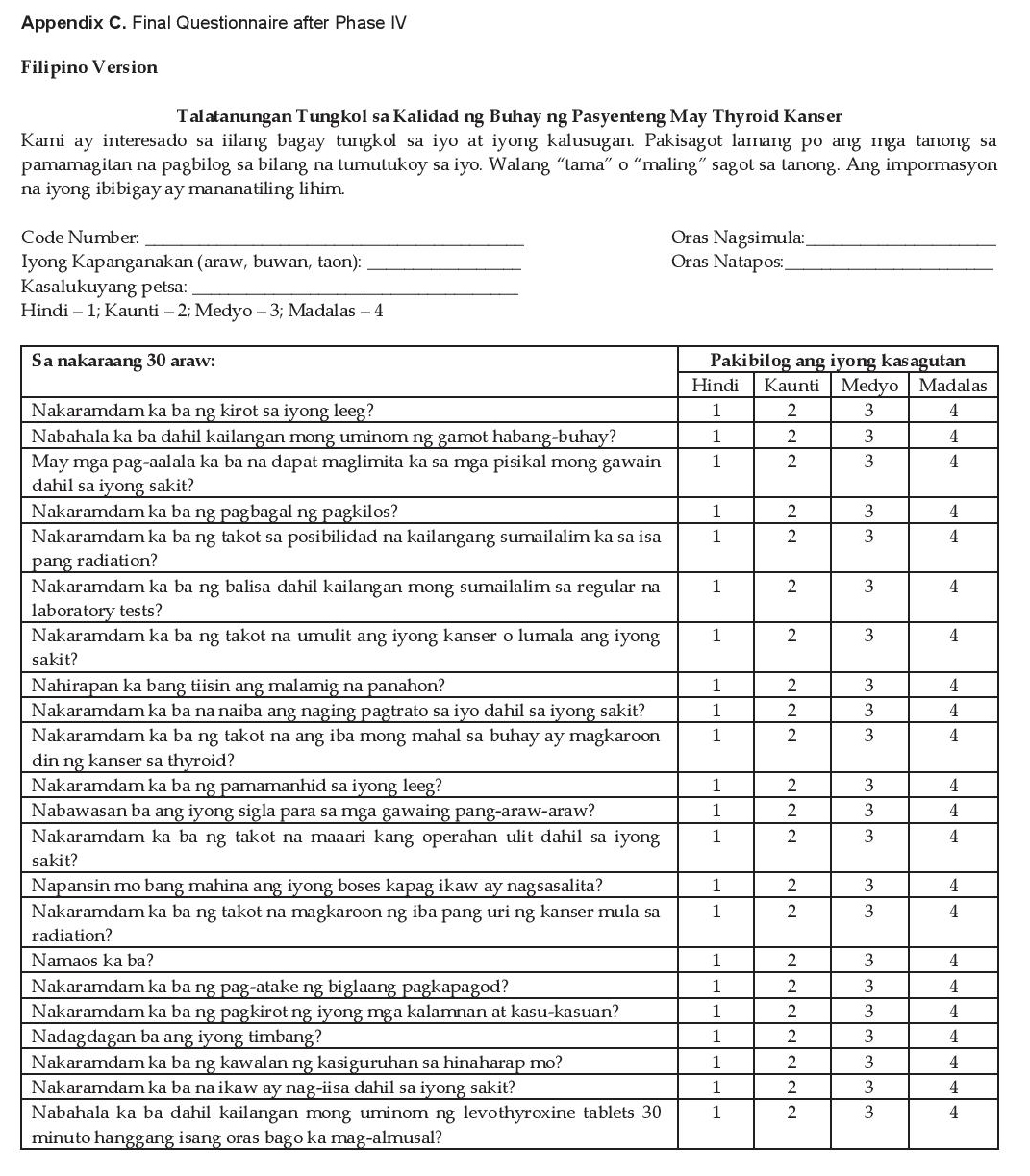
Because of the usually good prognosis and prolonged life of thyroid cancer patients compared to other cancer patients, using the final questionnaire (see Appendix C for the Filipino and English versions of the final questionnaire) to assess their quality of life is of value for physicians to guide therapy. Care of these patients can be improved based on their perceived HRQoL.
Click here to download Appendix CAppendix C. Final Questionnaire after Phase IV (Filipino Version)
Click here to download Appendix C
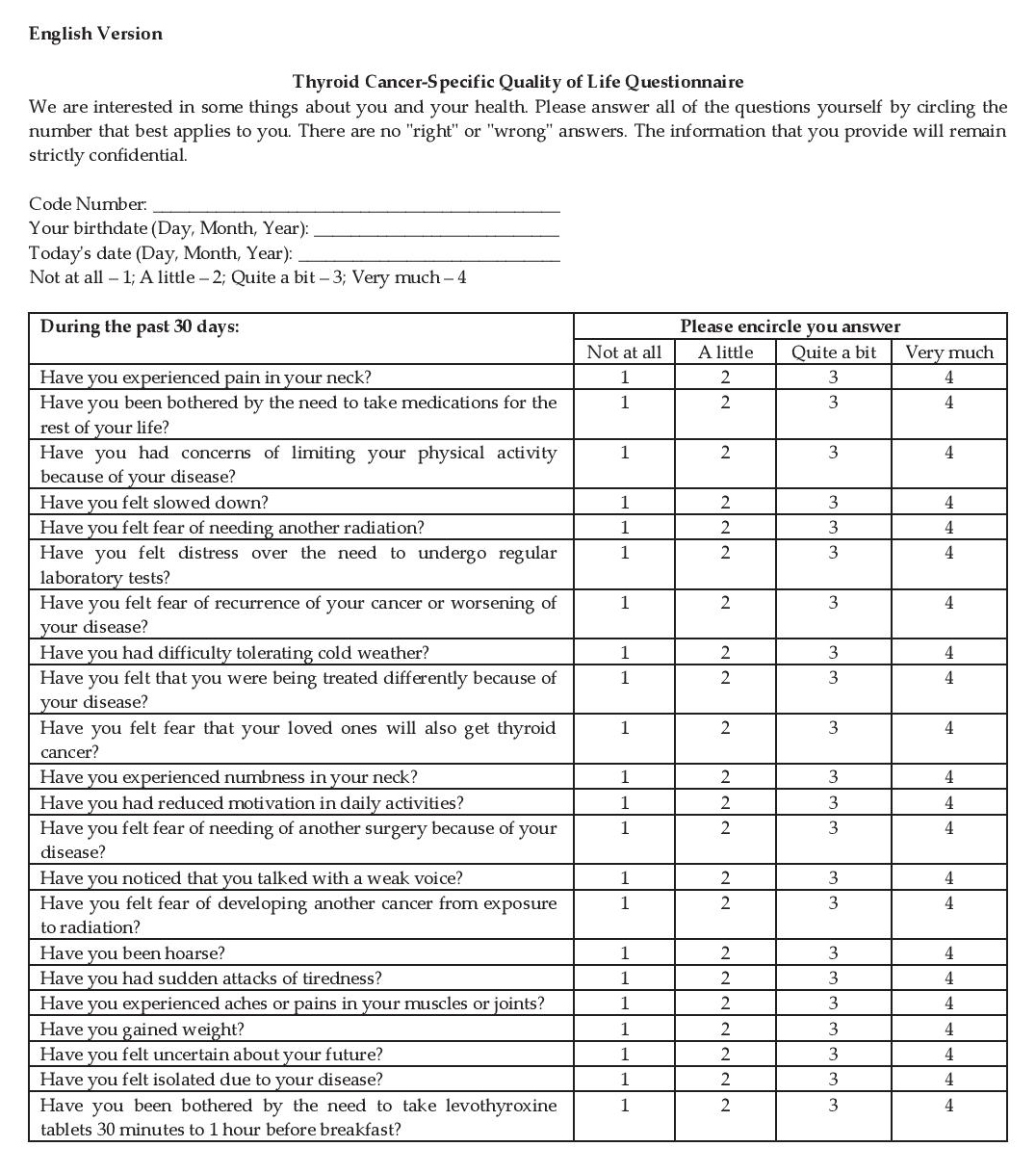
Appendix C. Final Questionnaire after Phase IV (English Version)
A 22-item questionnaire to assess HRQoL specific for Filipinos with DTC that can be used in combination with the EORTC QLQ-C30 was developed and validated. Five scales (perceived fear, psychological distress/anxiety, functionality, neck complaints, voice complaints) with good reliability, and acceptable convergent and divergent validity were identified.
AcknowledgementsThe authors are grateful to Professor Laurie Ramiro, a social scientist from the College of Arts and Sciences of the University of the Philippines Manila, who provided advice and suggestions critical for Phase II of the study. The invaluable comments of the consultants of the Section of Endocrinology, Diabetes and Metabolism of the Philippine General Hospital also helped improve and refine this study.
Statement of AuthorshipAll authors have given approval to the final version submitted.
Author DisclosureAll the authors have declared no conflict of interest to the work carried out in this paper.
Funding SourceThe Philippine Society of Endocrinology, Diabetes and Metabolism provided a monetary grant instrumental to the completion of the study.
[1] Curado MP, Edwards B, Shin HR, et al (eds). Cancer Incidence in Five Continents, vol. IX, IARC Sci Publ No. 160. Lyon, France: International Agency for Research on Cancer, 2007. Available from: http://www.iarc.fr/en/publications/pdfs-online/epi/sp160/CI5vol9.pdf. Accessed April 2015.
[2] Redaniel MTM, Laudico AV, Lumague MRM, Mapua CA, Patama T, Pukkala E. Cancer in the Philippines, vol. IV, part 1 - Cancer Incidence 1998-2002. Manila: Philippine Cancer Society, 2008. Available from: http://www.philcancer.org.ph/wp-content/uploads/2014/07/Cancer-in-the-Philippines-Vol.-IV-part-1.pdf. Accessed April 2015.
[3] Howlader N, Noone AM, Krapcho M, et al. SEER Cancer Statistics Review, 1975-2012, National Cancer Institute, Bethesda, MD. http://seer.cancer.gov/csr/1975_2012/. Accessed April 2015.
[4] Almeida, JP, Vartanian, JG, Kowalski, LP. Clinical predictors of quality of life in patients with initial differentiated thyroid cancers. Arch Otolaryngol Head Neck Surg. 2009;135(4):342-6. DOI.
[5] Rubic M, Kuna SK, Tesic V, Samardzic T, Despot M, Huic D. The most common factors influencing on quality of life of thyroid cancer patients after thyroid hormone withdrawal. Psychiatr Danub. 2014;26(Suppl 3):520-7. PubMed.
[6] Gómez MMN, Gutiérrez RMV, Castellanos SAO, Vergara MP, Pradilla YKR. Psychological well-being and quality of life in patients treated for thyroid cancer after surgery. Terapia Psicologica. 2010:28(1):69-84. DOI.
[7] Centers for Disease Control and Prevention. Measuring healthy days: Population assessment of health-related quality of life. Atlanta, Georgia: US Department of Health and Human Services, Centers for Disease Control and Prevention, 2000. Available from: https://www.cdc.gov/hrqol/pdfs/mhd.pdf. Accessed April 2015.
[8] Husson O, Haak HR, Oranje WA, Mols F, Reemst PHM, van de Poll- Franse LV. Health-related quality of life among thyroid cancer survivors: A systematic review. Clin Endocrinol (Oxf). 2011;75(4):544– 54. DOI.
[9] Aaronson NK, Ahmedzai S, Bergman B, et al. The European Organization for Research and Treatment of Cancer QLQ-C30: A quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst. 1993;85(5):365–76. DOI.
[10] Husson O, Haak HR, Mols F, et al. Development of a disease-specific health-related quality of life questionnaire (THYCA-QoL) for thyroid cancer survivors. Acta Oncol. 2013;52(2):447-54. DOI.
[11] Johnson, C, Aaronson N, Blazeby JM, et al. EORTC Quality of Life Group Guidelines for Developing Questionnaire Modules, 4th ed, April 2011. Available from: http://groups.eortc.be/qol/sites/default/files/ archives/guidelines_for_developing_questionnaire-_final.pdf. Accessed April 2015.
[12] Nunnally JC, Bernstein IH. Book review: Psychometric theory. 3rd ed. New York: McGraw-Hill, 1994.
[13] Jeong Y, Choi J, Ahn A, et al. Validation of the Korean version of the thyroid cancer-specific quality of life questionnaire. Ann Surg Treat Res. 2015;89(6): 287–294. http://dx.doi.org/10.4174/astr.2015.89.6.287.
[14] Dow KH, Ferrell BR, Anello C. Quality-of-life changes in patients with thyroid cancer after withdrawal of thyroid hormone therapy. Thyroid. 1997;7(4):613–9. DOI.
[15] Singer S, Andry G, Araújo C, et al. EORTC QOL Module for Thyroid Cancer (QLQ-THY). Available from: http://groups.eortc.be/qol/eortc-qol-module-thyroid-cancer-qlq-thy. Accessed April 2015.
Authors are required to accomplish, sign and submit scanned copies of the JAFES Author Form consisting of: (1) the Authorship Certification that the manuscript has been read and approved by all authors, and that the requirements for authorship have been met by each author, (2) the Author Declaration that the article represents original material that is not being considered for publication or has not been published or accepted for publication elsewhere, (3) the Statement of Copyright Transfer[accepted manuscripts become the permanent property of the JAFES and are licensed with an Attribution-Share Alike-Non-Commercial Creative Commons License. Articles may be shared and adapted for non-commercial purposes as long as they are properly cited], (4) the Statement of Disclosure that there are no financial or other relationships that might lead to a conflict of interest. For Original Articles involving human participants, authors are required to submit a scanned copy of the Ethics Review Approval of their research. For manuscripts reporting data from studies involving animals, authors are required to submit a scanned copy of the Institutional Animal Care and Use Committee approval. For Case Reports or Series, and Images in Endocrinology, consent forms, are required for the publication of information about patients. Articles and any other material published in the JAFES represent the work of the author(s) and should not be construed to reflect the opinions of the Editors or the Publisher.