Diabetes mellitus (DM) is a metabolic disorder characterized by hyperglycemia.[1] One of its disabling complications is sensory neuropathy including hearing loss. Sensorineural hearing loss (SNHL) is one form of hearing loss, which is caused by damage in the inner ear, involving the cochlea and its haircells.[2] Diabetes is thought to be an important causative factor for SNHL. It was described in one retrospective study that SNHL was more common in patients with diabetes than those without the disease.[3] The pathogenesis involves oxidative stress, microangiopathy and auditory neuropathy.[4] The prevalence of SNHL in diabetics varies widely in different studies and the data regarding its association with glycemic control are inconsistent. Studies on different populations have shown a prevalence range of 21.7% to as high as 73.3%.[5]-[7] These results support the finding that the prevalence of SNHL varies among different racial ethinicities.[8]A few studies tried to determine the association of SNHL with the degree of glycemic control. Fasting blood sugar was noted to be correlated with the severity of SNHL, however conflicting results have been noted in the association of hearing loss and HbA1c levels.[7]-[9]
According to the Philippines’ latest National Health and Nutrition Survey (NNHeS) in 2013, the prevalence rate of diabetes by oral glucose tolerance test is 5.4%.[10] However there is no local data available describing the prevalence of SNHL in Filipino patients with diabetes. It seems that SNHL is a less recognized complication of diabetes.
The World Health Organization (WHO) defines hearing impairment as pure-tone thresholds of more than 25 dB hearing loss in the better ear.[11] Hearing loss may be mild (threshold of 26-40 dB), moderate (threshold of 41-55 dB), moderately severe (threshold of 56-70 dB), severe (threshold of 71-90 dB), and profound (threshold of >90 dB).[12] Disabling hearing loss refers to thresholds greater than 40 dB in the better ear. Above this threshold, hearing impairment makes it difficult to hear speech sounds lower than normal voices and may cause individuals to miss parts of or all of the words in ordinary conversation.[11] Hearing loss is thus considered a disabling problem.
This study aims to ascertain the prevalence of SNHL among adult Filipinos with diabetes consulting in a tertiary urban hospital. It also aims to determine the association between the presence of SNHL and poor glycemic control.
METHODOLOGYA cross-sectional analytic study was done among 128 patients with diabetes mellitus consulting at the outpatient departments of Philippine General Hospital, a tertiary hospital in Manila. Included were adults aged 19 and above, with Type 1 or Type 2 diabetes mellitus with disease duration of at least five years, diagnosed with the disease based on Philippine practice guidelines for diagnosis and management of diabetes and with at least one HbA1c result per year during the last five years.[12] Exclusion criteria were presence of risk factors for SNHL such as history of ototoxic drug exposure (aminoglycosides, loop diuretics such as furosemide, salicylates, and chemotherapeutic drugs) within the last 5 years, history of radiation exposure in the head within the last 5 years, occupational noise exposure, congenital/structural deformity in the ear, abnormal otoscopy findings, conductive hearing loss of unknown etiology, and infections (otitis media, syphilis, herpes zoster). Those with conditions that preclude accurate pure tone testing such as claustrophobia were also excluded.
Using Epi Info version 7, the minimum sample size requirement is at least 128 based on the prevalence of SNHL in this population=21.7% with 95% confidence level, 7.5% margin of error, and 10% non-response rate (possibility of incomplete patient charts during review of records).[13] Stratified random sampling was done to recruit patients from the family medicine, internal medicine, and diabetes clinics. The three different clinics were used as strata. The list of patients scheduled for consult for the day was used as the sampling frame. Proportionate allocation was implemented in recruiting patients from each stratum.
We randomly selected 15 patients from the family medicine clinic, 24 from the internal medicine clinic, and 89 from the diabetes clinic. Patients who met the inclusion criteria and gave their informed consent were included in the study. If the invited subject declined, then he or she was replaced by randomly selecting a patient consulting in that clinic. The University of the Philippines Manila Research Ethics Board approved the study for implementation.
The following data were collected from each patient by interview and physical exam: date of birth, approximate date (nearest month and year) of diagnosis of diabetes. Weight (kilograms) and height (centimeters) were obtained using a standard weighing scale with stadiometer without shoes. Systolic and diastolic blood pressures were obtained uniformly using the right arm with the subject seated and using a digital sphygmomanometer. A blood pressure ≥140/90 is considered hypertensive, and below that level, nonhypertensive. Chart review was done to retrieve the HbA1c values for the last five years, and then the average HbA1c was computed. An average of <7% was classified as controlled diabetes, and an average of ≥7% was classified as uncontrolled diabetes.
Click here to download Appendix AAppendix A.Data Collection Forms

The presence of diabetic complications in each participant was determined by reviewing chart data. The presence of albuminuria was obtained from the latest urinalysis within a year from inclusion. The most recent creatinine during the latest consult was used to calculate the creatinine clearance using the Cockroft and Gault formula. The presence of retinopathy was based on the most recent consult with the ophthalmologist within the past year. Peripheral neuropathy was screened using the Neuropathy Disability Score (NDS).
Recruited patients underwent PTA-ST using the Madsen Itera II Diagnostic Audiometer at the Ear Unit of the Philippine General Hospital. These machines were ISO certified and are regularly calibrated. Air conduction and bone conduction were tested. The threshold expressed in decibels (dB) were measured for sound frequencies 250, 500, 1000, 2000, 4000, and 8000 hertz (Hz) for air conduction and 500, 1000, 2000, and 4000 Hz for bone conduction. The average of the air conduction threshold for the 500, 1000, and 2000 Hz was used to determine the pure tone average. The presence of a threshold of PTA >25 db in at least one ear, with bone conduction average not more than 10db from the PTA is diagnostic of SNHL.
Data AnalysisData analysis was performed using STATA version 13 statistical software. Quantitative variables were presented as mean and standard deviation, while qualitative variables were presented as frequency and percentage. Logistic regression analysis was done to analyze factors associated with SNHL among patients with diabetes. Independent predictors of SNHL among patients with diabetes were determined using multiple logistic regression analysis with backward elimination. The level of significance was set at 5%.
A total of 148 patients were recruited to participate in the study, however 20 were excluded due to a history of chemotherapy, history of exposure to loud noises, impacted cerumen, presence of ear infection, and no consent. There were 128 patients eligible for the study. The clinical and demographic characteristics of all participants are shown in Table 1. These individuals underwent PTAST. Data on the presence of diabetic complications were gathered. Eight patients had no data on albuminuria, three patients did not have creatinine values, and six patients did not have screening for retinopathy at the time of inclusion in the study.
Click here to download Table 1Table 1.Demographic and clinical profile of all participants. (n=128)
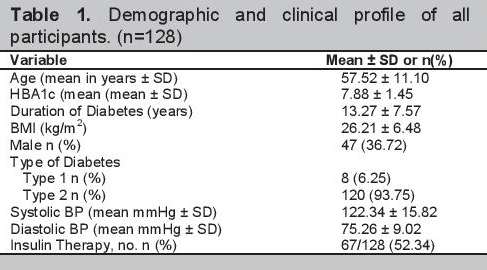
Among the 128 patients included in the study, 58 or 45.31% had SNHL. Twenty-one patients had unilateral SNHL and 37 patients had bilateral SNHL. Among those with bilateral SNHL, 26 patients had symmetric SNHL and 11 patients had asymmetric SNHL. Among patients who had SNHL, 41 patients had mild hearing loss, 12 had moderate hearing loss, 4 patients had moderately severe hearing loss, none had severe hearing loss, and 1 had profound hearing loss. The demographic and clinical characteristics of the participants with SNHL and without SNHL are compared in Table 2.
Click here to download Table 2Table 2.Association of demographic and clinical characteristics of patients with sensorineural hearing loss, (N=128)
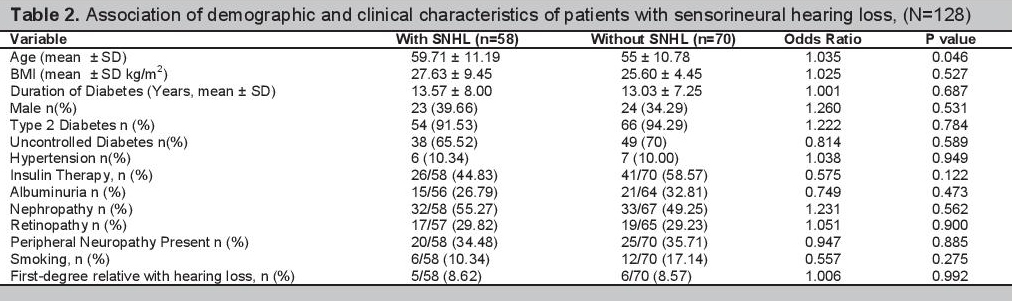
There were 87 (70.16%) participants with a five-year average HbA1c ≥7%. Among these patients, 38 (43.68%) had SNHL and 49 (56.32%) did not have SNHL. The proportion of patients with SNHL with a five-year average HbA1c <7% compared to those patients with a five-year average HbA1c ≥7%, were 48.78% (20/41) and 43.68% (38/87) respectively, p value=0.588.
There were 23 (33.33%) patients age 60 years old and below who had SNHL and 35 (59.32%) patients above 60 years old who had SNHL (p value=0.003). Among the 47 males, 23 (48.94%) had SNHL and among the 81 females, 35 (43.21%) had SNHL (p value=0.530).
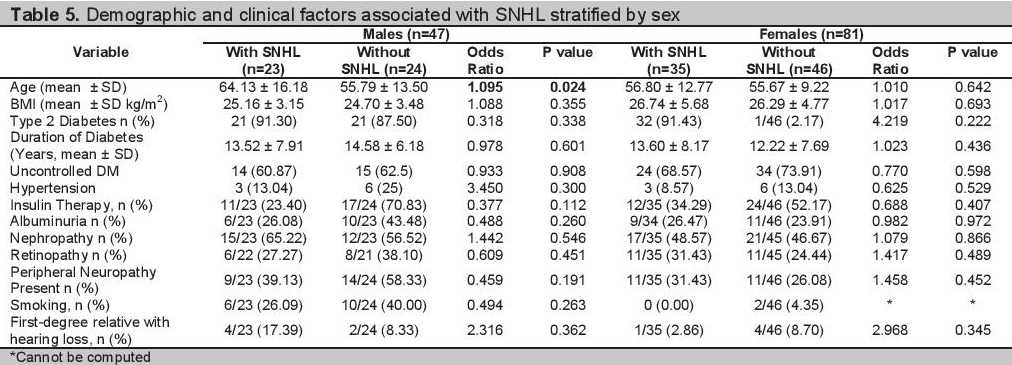
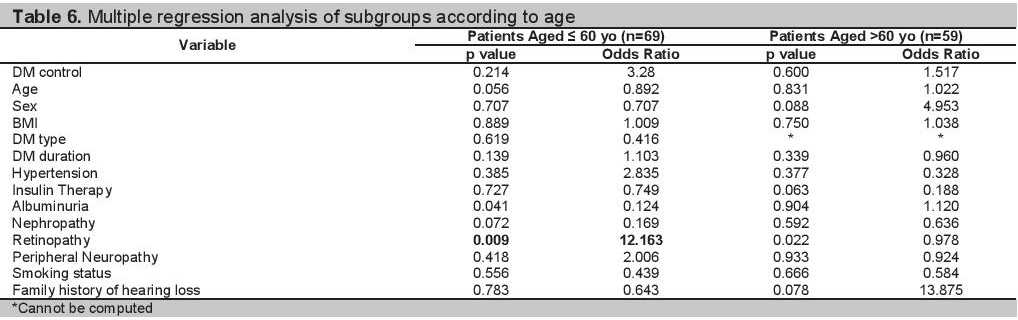
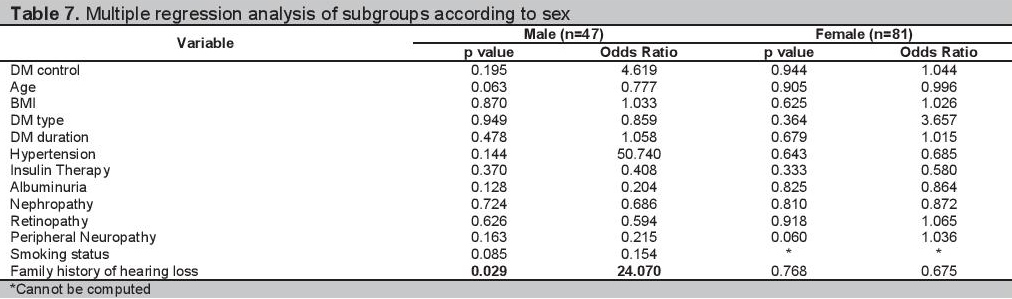
Multiple logistic regression analyses of the 128 participants are shown in Table 3. The clinical and demographic profiles of the participants grouped according to age and sex are shown in Tables 4 and 5 respectively. The multiple regression analysis according to age group and sex are shown in Tables 6 and 7.
Click here to download Table 3Table 3.Multiple Logistic Regression Analysis (N=128)
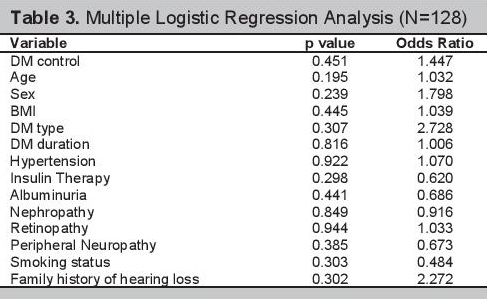
Click here to download Table 4
Table 4.Demographic and clinical factors associated with SNHL stratified by age
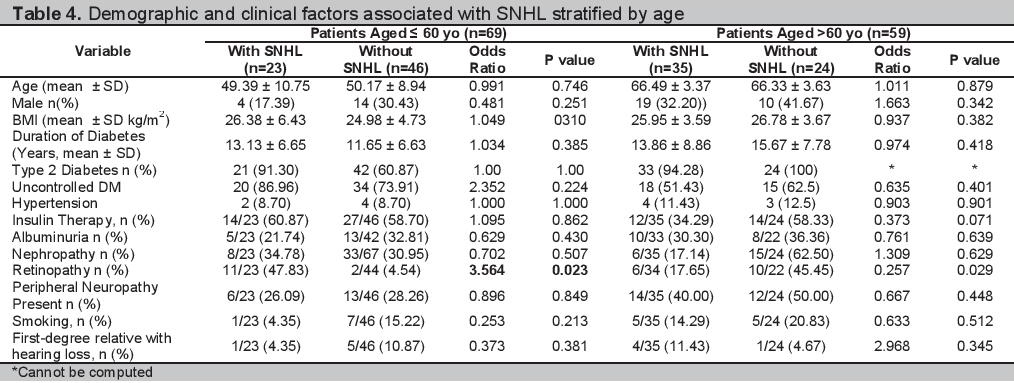
Click here to download Table 5
Table 5.Demographic and clinical factors associated with SNHL stratified by sex
Click here to download Table 6
Table 6.Multiple regression analysis of subgroups according to age
Click here to download Table 7
Table 7.Multiple regression analysis of subgroups according to sex
Prevalence of Sensorineural Hearing Loss and Clinical Characteristics of Participants
Almost half of the patients included in our study have hearing loss. The observed prevalence rate of SNHL in our hospital is 45.31%, which is within the range of the observed prevalence rates in different studies.[6][7] It is very similar to the reported 44% rate in a study done in Turkey and 45% prevalence rate in a study done in Iran.[4][14]
Among the demographic and clinical characteristics of patients investigated in our study, mean age was the only variable that was associated with SNHL (p=0.046, OR 1.035). The group with SNHL tended to be older than those without SNHL (Table 2). A more advanced age seemed to be associated with the presence of SNHL. This might be due to the development of presbycusis, since the risk for hearing loss increases steadily with increasing age.[15]
The groups of patients with SNHL and without SNHL are similar in terms of diabetes control, BMI, presence of hypertension and diabetes duration. The two groups are also comparable in terms of proportion of males, insulin treatment, smoking history and family history of hearing loss. The presence of diabetic complications such as retinopathy, nephropathy and retinopathy also does not seem to be associated with having SNHL. There was no statistically significant difference in the presence of these complications in both groups of patients (Table 2). The results are consistent with the findings of De Leon- Morales among 94 patients with diabetes, wherein the presence of SNHL is independent of the presence of other diabetic complications such as peripheral neuropathy, retinopathy and neuropathy.[16] However, these results are different from the large retrospective study done by Kakarlapudi, where patients with increasing creatinine correlated with progressive hearing loss.[3]
Other factors have been described in literature to be associated with SNHL such as hypertension and duration of diabetes. It was described that a higher prevalence of SNHL is observed in patients with higher blood pressure[17][18] In our study, patients with SNHL did not have higher blood pressure levels than those who did not have SNHL. Diabetes duration was also not statistically significant between those patients with SNHL and without SNHL. Our finding is consistent with the findings of Rajendran, where there was a higher prevalence of SNHL which was not associated with longer diabetes duration. This finding however is in contrast with the results of the study of Mozzafari and Austin.[6][14][20]
Sensorineural Hearing Loss and Glycemic ControlTwo different studies described the association of SNHL with fasting blood sugar level (FBS). One study showed a statistically significant higher rate of SNHL among patients with elevated FBS levels and another also showed a higher proportion of patients with SNHL among patients with elevated FBS levels but was not statistically significant.[9][10] These studies used FBS instead of HbA1c in determining glycemic control, which is the ideal test in assessing blood sugar control. One study used HbA1c level in assessing glycemic control showed a positive correlation of SNHL and glycemic control. The study showed a higher prevalence of SNHL in patients with higher HbA1c level. Those with SNHL had an average HbA1c of 12.2±3.2% and those without SNHL had an average HbA1c of 9.8±2.6% with a p value of 0.02.[7] This study however included only 46 patients with diabetes and correlated SNHL with only a single, most recent HbA1c result. Our study included a bigger number of patients and correlated the presence of SNHL with the average glycemic control over a longer period of time. Those with SNHL had a five-year average HbA1c of 7.78±1.51 and those without SNHL had a five-year average HbA1c of 7.96±1.41. There was no statistically significant difference in the HbA1c levels in both groups with a pvalue of 0.476. The results of our study show that there seems to be no association of long term glucose control with the presence of SNHL.
Multiple logistic regression analysis with backward elimination was done to determine independent predictors of SNHL among all the 128 patients recruited. However, none of the clinical characteristics investigated in our study are independent predictors of SNHL. After the multiple logistic regression analysis, age does not seem to be a predictor of SNHL among our patients, p value=0.195 (Table 3).
Analysis by AgeIn our study there were more patients with SNHL in the age group above 60 years old compared to the age group of 60 years old and below (p value=0.003). Therefore, segregating the results according to age might help lessen the effect of presbycusis. Simple bivariate analysis of the presence of SNHL and diabetes control in patients age 60 years old and below, showed no association, as well as in the age group above 60 years old with p values of 0.224 and 0.401 respectively (Table 4). Our results show that there seems to be no association of the presence of SNHL and long-term glycemic control regardless of age.
Those patients with SNHL in the age group 60 years old and below had a greater proportion of retinopathy than those who did not have SNHL. The difference between the two groups is significant (p value of 0.023 and OR of 3.564). However, in the age group above 60 years old, there were more patients without SNHL who had retinopathy, than those patients with SNHL who had retinopathy (p value of 0.029). After multiple regression with backward elimination analysis, retinopathy was not an independent predictor of SNHL (Table 6). There were no other variables that are predictors of SNHL among patients 60 years old and below and those above 60 years old.
Analysis by SexIn our study there was no difference in the proportion of males and females with SNHL. Our findings are consistent with the studies of Rajendran and Srinivas, wherein the association of hearing loss in patients with diabetes and sex is insignificant.[6][21] In the male population, a more advanced age was associated with SNHL (Table 5). However on multiple regression analysis, it was a family history of hearing loss that is an independent predictor of SNHL with a p value=0.047, OR=1.088 (Table 7). Among females, there were no clinical factors that have been identified to be associated with having SNHL in the bivariate and multiple regression analysis (Table 5 and Table 7).
It seems that patients with diabetes are at risk of having SNHL regardless of their blood sugar control. Limiting exposure to ototoxic drugs and loud noises may help decrease the risk of having SNHL among patients with diabetes. Screening for SHNL may be necessary so appropriate treatment maybe given since hearing loss is a disabling complication that may significantly affect the quality of life of patients with diabetes.
There is a high prevalence rate of SNHL among Filipino patients consulting in our hospital. There seems to be no association between the presence of SNHL and long-term glycemic control. Therefore patients with diabetes are at risk of having SNHL regardless of blood sugar control. Among the clinical variables investigated in our study, a family history of hearing loss increases the odds of having SNHL among male patients with diabetes. Screening for SNHL among patients with diabetes may be warranted, regardless of blood sugar control, especially in patients with age 60 years old and below with retinopathy. Hearing care, focusing on prevention of hearing loss should be advocated for patients with diabetes mellitus.
AcknowledgmentsWe would like to recognize Maria Luz M. San Agustin RN, audiometrician at the Philippine National Ear Institute, National Institutes of Health, for performing the PTA on all the participants.
Statement of AuthorshipAll authors have given approval to the final version submitted.
Author DisclosureAll the authors have declared no conflict of interest to the work carried out in this paper.
Funding SourceNone.
[1] Stumvoll M, Goldstein BJ, van Haeften TW. Type 2 diabetes: Principles of pathogenesis and therapy. Lancet. 2005; 365(9467):1333– 46. DOI.