A 46-year old female presented to our hospital in 2008 with gradual onset of paraparesis of both lower limbs. She had right hemi-thyroidectomy performed 12 years ago at another hospital. She did not monitor TSH, nor did she receive levothyroxine replacement at any time. Magnetic resonance imaging (MRI) of the spine showed multiple areas of abnormal marrow signals in the spine, involving the T4, T6, T7, T12, L2, L3 and S1 vertebrae. Collapse of the T4 vertebra was seen with posterior cortical margin convexity and associated pre- and para-spinal and anterior epidural soft tissue components, causing compression on the thecal sac with moderate to severe secondary central canal stenosis (Figures 1A and B). Computed tomography (CT)-guided biopsy of the third lumbar spinal lesion revealed tumor cells which were positive for thyroglobulin immunohistochemistry, suggestive of metastatic follicular thyroid carcinoma.
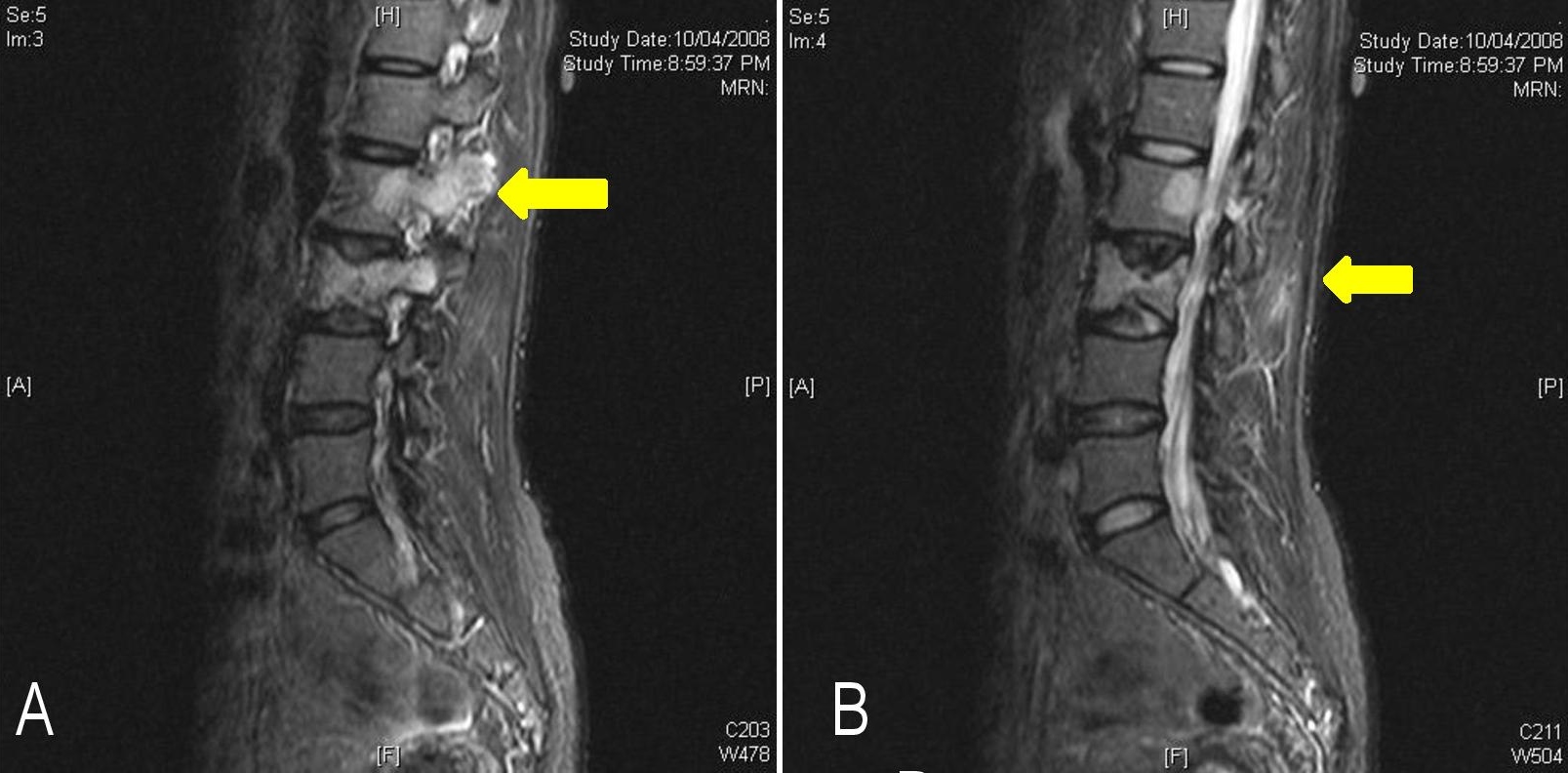
Figure 1. Magnetic resonance imaging of the spine showing vertebral metastases (yellow arrow) on parasagittal (A) and sagittal (B) views.
CT scan of the neck showed a remaining homogenous left thyroid gland with no abnormal calcification or nodule. A right-sided pleural effusion with collapse and consolidation of the right lower lobe of the lung was also present. Pleural fluid biochemistry was exudative, but cytology showed nonspecific staining of mesothelial cells and macrophages. It was most likely malignant in origin. Serum thyroglobulin was markedly elevated (3682 mg/mL) and antithyroglobulin antibody was <4 IU/mL.
She refused total thyroidectomy or any surgical intervention for her spinal compression. Local radiotherapy to the spine was then given. As radiotherapy was done in another hospital, we were not aware of the response to treatment. Bisphosphonate therapy was also not given. Due to the extent of disease, palliative care was offered. She defaulted clinic follow-up and was admitted several times for recurrent pleural effusion. The event leading to death 2 years ago was respiratory failure, which may have been due to pulmonary embolism due to chronic immobilization.
Serial thyroid tests showed extremely low fT4 levels with normal fT3, TT3 and TSH. Levothyroxine 100 mcg daily was started, which raised fT4 minimally but increased fT3 to 1.5-fold the upper limit of normal and suppressed TSH even further (Table 1). Cortisol showed appropriate response to Synacthen®: baseline serum cortisol of 749 nmol/L rose to 1236 nmol/L at 30 minutes and 1447 nmol/L at 60 minutes. Adrenocorticotropic hormone, follicular stimulating hormone, luteinizing hormone, estradiol and prolactin were normal.
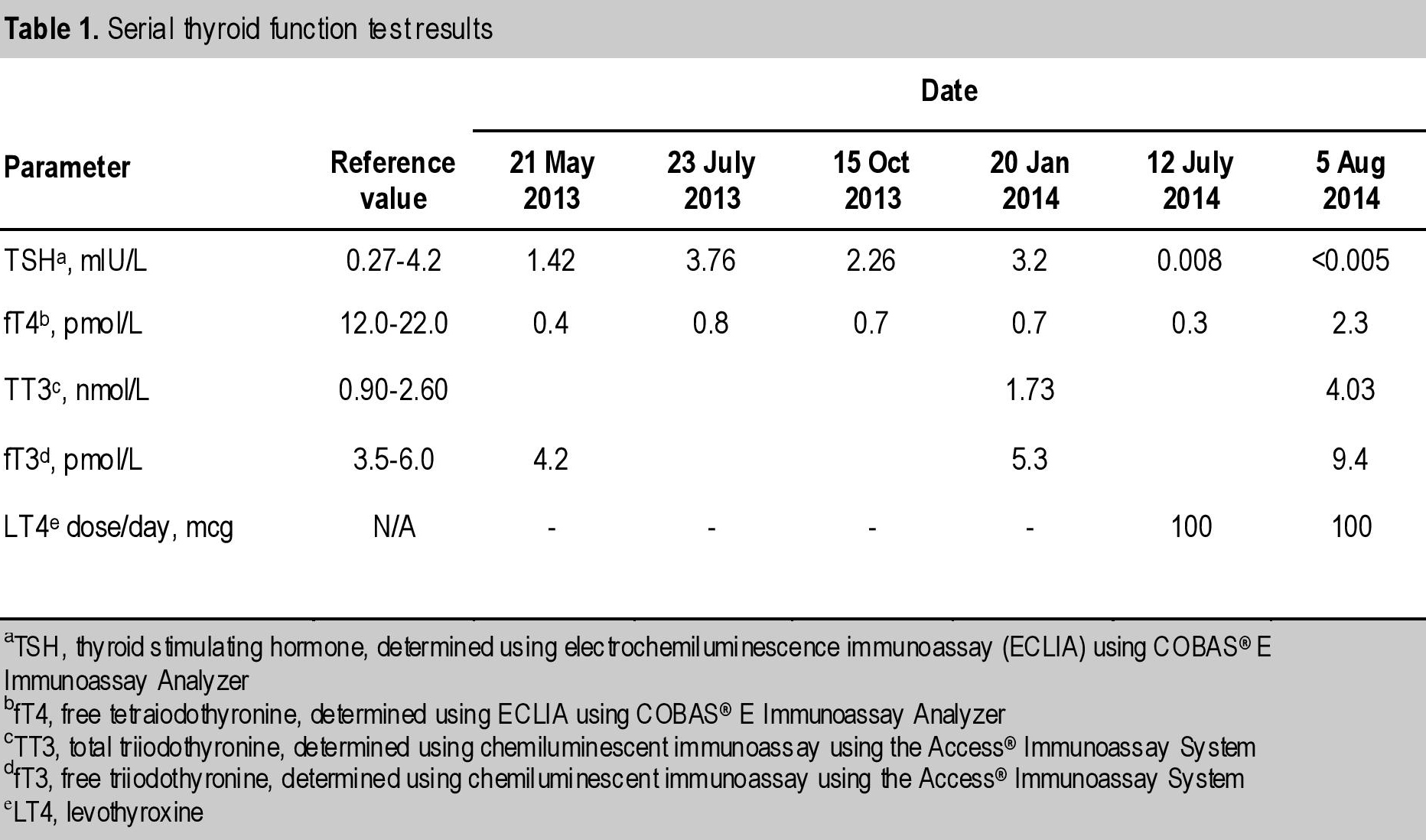
Table 1. Serial thyroid function test results
Thyroid hormone production is regulated by both pituitary and peripheral factors. While the secretion of TSH is inhibited by T4 and T3, it is stimulated by thyrotropin releasing hormone. The thyroid follicular epithelial cell is responsible for the biosynthesis of thyroid hormones. As the main product of the thyroid gland, T4 functions as a prohormone which is transformed into the biologically active hormone, T3. Extra-thyroidal conversion of T4 to T3 is regulated by nutritional, hormonal and illness-related factors. Approximately 80% of the T3 produced is formed by 5’-deiodination of T4 in extra-thyroidal tissue.[1] Three iodothyronine deiodinases (D1, D2, D3) have been identified.
T4 and TSH are usually undisturbed in thyroid carcinoma. However, in a minority of cases of metastatic follicular thyroid carcinoma, an elevated serum T3 to T4 ratio is seen. Several studies have evaluated the expression of deiodinase activity in thyroid neoplasms. D1 activity has been found to be reduced in papillary, follicular and anaplastic thyroid carcinomas. In contrast, a high level of D2 activity has been reported in metastatic follicular thyroid carcinoma, resulting in an increased fractional conversion of T4 to T3.[2],[3],[4]
D1 and D2 activities were assayed in the tumor resected from a patient in a previous case report.[2] The tumor D2 converted 73 ± 3% of T4 to T3, compared to 26 ± 3% in the normal human thyroid. Most of these patients presented with either bulky primary tumors or metastatic disease. Akira et al., found that 20% of patients with massive metastatic follicular carcinoma had T3 thyrotoxicosis while on thyroxine treatment, whereas none of the patients with papillary or medullary thyroid carcinoma exhibited this phenomenon.[5]
Our patient had very low serum fT4, in the range of 0.4 to 0.7 pmol/L, with normal levels of TSH, fT3 and TT3. When exogenous thyroxine was started, fT4 increased marginally, with a consequent suppression of TSH and increase in fT3 and TT3. This is an indirect evidence of hyperconversion of T4 to T3, likely due to a high D2 activity. However, the activity of deiodinase was not evaluated in the tumor tissue of our patient.
In these patients, frequent monitoring of T3 levels along with fT4 and TSH should be done to avoid overzealous treatment with exogenous thyroxine. Unfortunately, we were not able to titrate and optimize the dose of levothyroxine in this patient, as she defaulted follow-up and only returned later with other complications.
It is rational to consider elevated D2 deiodinase activity in follicular thyroid carcinoma as a cause of a low T4 with normal TSH. This increases the conversion of T4 to triiodothyronine, making measured T3 levels normal. Thyroid suppression therapy should be titrated according to T3 and TSH levels to avoid T3 thyrotoxicosis.
Ethical ConsiderationConsent from the patient was not obtained because of her demise.
Statement of AuthorshipAll authors certified fulfillment of ICMJE authorship criteria.
Author DisclosureThe authors have declared no conflict of interest.
Funding SourceNone.
[1] Bianco AC, Salvatore D, Gereben B, Berry MJ, Larsen PR. Biochemistry, cellular and molecular biology, and physiological roles of the iodothyronine selenodeiodinases. Endocr Rev. 2002;23(1):38-89. PubMed DOI.