Diabetic kidney disease (DKD), or diabetic nephropathy (DN), is a major microvascular complication of diabetes mellitus (DM) and is now the leading cause of end-stage renal disease (ESRD) worldwide.[1] It affects 30-40% of Type 1 DM patients, usually 20-25 years after disease onset, as well as an approximate number of Type 2 DM patients after a variable number of years.[2] The pathogenesis and clinical stages of DKD appear to be similar for both types of DM.[3] Kidney damage initially manifests with renal hypertrophy and hyperfiltration, eventually progressing to microalbuminuria with increased urinary albumin excretion rates (UAER) of 30-300 mg/day. Microalbuminuria is the earliest clinically detectable stage of DKD at which appropriate intervention can delay or reverse the disease process.[4] Without proper intervention, 20-40% of microalbuminuric patients progress to macroalbuminuria with UAER exceeding 300 mg/day; of these, 20% eventually reach ESRD in their lifetime.[1] As both micro- and macroalbuminuria are powerful risk factors for cardiovascular disease – another leading cause of mortality and morbidity in DM – the importance of intensive efforts to prevent and treat DKD is justified.[5]
Primary prevention of DKD is attained in patients with normal kidney function through strict glycemic and blood pressure (BP) control, preferably employing reninangiotensin- aldosterone system (RAAS) modulating drugs such as angiotensin-converting enzyme inhibitors (ACEI) and angiotensin II receptor blockers (ARB). Secondary prevention, on the other hand, aims to retard the progression from micro- to macroalbuminuria and likewise requires ACEI or ARB to achieve BP targets.[6] However, with worsening DKD, the efficacy of these interventions becomes less than optimal, often necessitating additional therapies to delay the progression of renal disease.[7]
The typical pathological changes in DKD involve glomerular basement membrane (GBM) thickening and mesangial expansion and proliferation, leading to nodular glomerulosclerosis and the formation of Kimmelstiel- Wilson lesions. Glycosaminoglycan (GAG) moieties – of which heparan sulfate is the most abundant – are also decreased in the diabetic GBM in proportion to the degree of proteinuria, mainly due to abnormalities in synthesis, sulfation, composition, and matrix interactions.[8] This explains the potential use of heparin derivatives, or heparinoids, as potentially useful anti-proteinuric drugs that could supplement RAAS modulating-treatment. They include sulodexide, an oral heparinoid with high concentrations in the renal parenchyma; conventional and low molecular weight heparin (LMWH); factor Xainhibitors such as danaparoid and fondaparinux; and animal-derived sources such as chondroitin, keratan, and dermatan sulfate.[9] Proposed mechanisms include restoration of GBM ionic permselectivity, prevention of GAG degradation, suppression of albuminuria-induced and endothelin-mediated inflammation, and inhibition of apoptosis in glomerular cells.[10],[11]
In streptozotocin-induced diabetic rats, sulodexide effectively lowered UAER, improved renal ultrastructure, and prevented GBM thickening, in addition to exerting direct endothelial protective effects.[12] In humans, the results were more equivocal. Some trials demonstrated reductions in UAER and serum creatinine (SCr) levels in sulodexide-treated groups, as well as improvements in HbA1c, BP, and lipid profile.[13],[14],[15],[16],[17] Other studies, however, failed to demonstrate renoprotective benefits, particularly in terms of the patient number achieving significant reduction or normalization of the urine albumin-creatinine ratio (ACR).[18],[19] Similarly, for other heparinoids, small trials showed conflicting data. Reductions in UAER were seen with enoxaparin but not with tinzaparin; for danaparoid, reductions occurred with Type 1 but not with Type 2 DM patients.[8],[20],[21],[22] Thus, the exact role of heparinoid supplementation in DKD remains unknown and these substances are not part of treatment recommendations. This study attempts to consolidate available information and evaluate their safety and efficacy in DKD patients.
METHODOLOGYSearch Strategy
Electronic databases including MEDLINE, Embase, Scopus, Herdin, ClinicalTrials.gov, Google Scholar, and the Cochrane Central Register of Controlled Trials were systematically searched by two independent investigators for eligible articles. For the intervention of interest, the following terms were used individually and in combination: “sulodexide,” “Vessel Due-F,” mucopolysaccharide,* proteoglycan,* syndecan,* galactosaminoglycan,* glycosaminoglycan,* glycoamin,* chondroitin,* keratin,* dermatan,* heparinoid,* heparin,* heparin,* hyaluron,* “low molecular weight heparin,” “LMWH,” ardeparin,* “Normiflo,” bemiparin,* “Hibor,” certoparin,* Sandoparin,* dalteparin,* enoxaparin,* “Clexane,” nadroparin,* Fraxiparin,* Seleparin,* parnaparin,* “Fluxum,” reviparin,* tedegliparin,* tedelparin,* tinzaparin,* “Innohep,” “danaparoid,” “fondaparinux,” “Arixtra,” “idraparinux”. For the disease of interest, the following search terms were used: diabetes,* diabetic,* “DM,” “IDDM,” “NIDDM,” kidney,* renal,* nephro,* nephriti,* glomerulo.* These key terms were utilized as text words, Medical Subject Headings (MeSH), and Clinical Queries. Cross-references of original publications, books of abstracts, and conference proceedings from the WHO Network of Collaborating Clinical Trial Registers, US FDA registry, and International Committee of Medical Journal Editors (ICMJE) were searched as well. Manufacturers were also contacted for possible unpublished studies.
Study SelectionTrials involving heparinoid supplementation to delay or prevent the progression of DKD were included. We included patients ≥18 years old, diagnosed with either Type 1 or Type 2 DM according to the American Diabetes Association 1997 criteria [fasting plasma glucose (FBS) ≥126 mg/dL or 2-hour plasma glucose ≥200 mg/dL after an oral glucose tolerance test], having either microalbuminuria (UAER 30-300 mg/day) or macroalbuminuria (UAER >300 mg/day). Those diagnosed with other forms of DM (i.e. gestational DM) and having contraindications to heparinoid use (i.e., pregnancy, deranged clotting parameters, bleeding diathesis, or thrombocytopenia) were excluded. There were no restrictions on ethnicity, language, or gender. Studies must utilize a heparinoid as the primary intervention, regardless of dosage, mode of administration, or duration of treatment, and on top of standard DKD therapy in terms of glycemic control and use of either an ACEI or ARB. The primary outcome measure is all-cause mortality rate. Secondary outcomes include parameters of disease progression such as changes in UAER, ACR, SCr, or creatinine clearance (CrCl), changes in patient number with reductions in the above parameters, rates of hospitalization or dialysis, time to ESRD, and changes in health-related quality of life.
Data Extraction and ManagementTwo authors independently screened the eligibility of studies. Studies agreed upon for exclusion by both reviewers were excluded at this stage, with the reason for exclusion documented. Eligible studies then underwent methodological quality assessment based on the Cochrane Collaboration's tool for assessing risk of bias. Any disagreements were resolved by a third author. Studies that passed all screenings underwent data extraction using a customized data extraction form. The following data were extracted from each of the included trials: author, year of publication, location of study, duration of study, intervention, comparator, sample size and type of population, and study outcomes.
Statistical AnalysisThe study was analyzed using Review Manager, version 5.1. Results were presented as mean differences (MD) and standard deviations (SD) with 95% confidence intervals, and graphically presented as forest plots. Estimates were calculated using the Mantel-Haenszel odds ratio for dichotomous variables and the inverse variance method for continuous variables. These were pooled using either the fixed or random effects model depending on heterogeneity (defined as I^2>50%). Heterogeneity, if present, was further explored using both sensitivity and subgroup analysis. For studies with multiple follow-up periods, data at the end of treatment or at maximum follow-up period were used. Unit of analysis issues were resolved by looking for uniformity among the analyses of the individual studies.
Search Results
Thirty-four potentially relevant articles were retrieved. On initial deliberation, only thirty were eligible for inclusion; the other four were excluded because they were either editorials or review articles. Of the thirty screened-in articles, eighteen were further excluded because they either had no active comparator or had different interventions, disease populations or outcome measures.
Twelve studies ultimately satisfied the selection criteria. Figure 1 shows the study selection flowchart, while Tables 1 and 2 show the list of included and excluded studies, respectively.
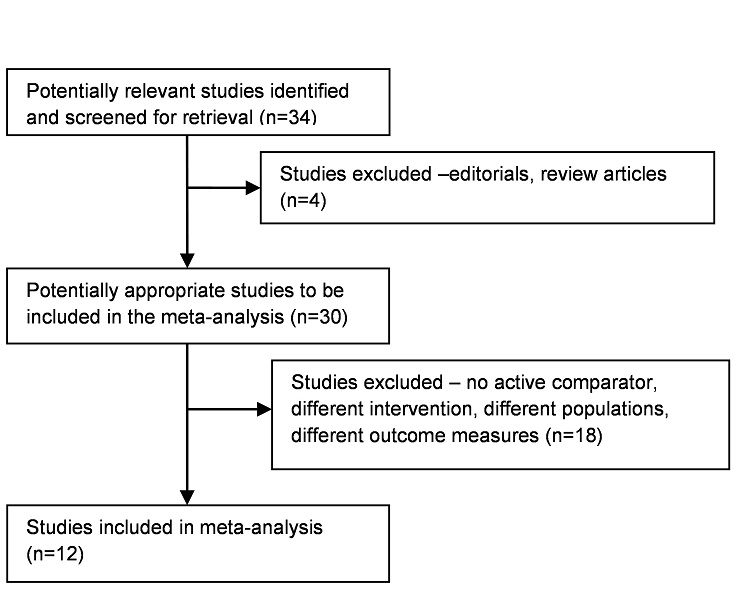
Figure 1. Flowchart of the process of retrieval and selection of studies for the meta-analysis.
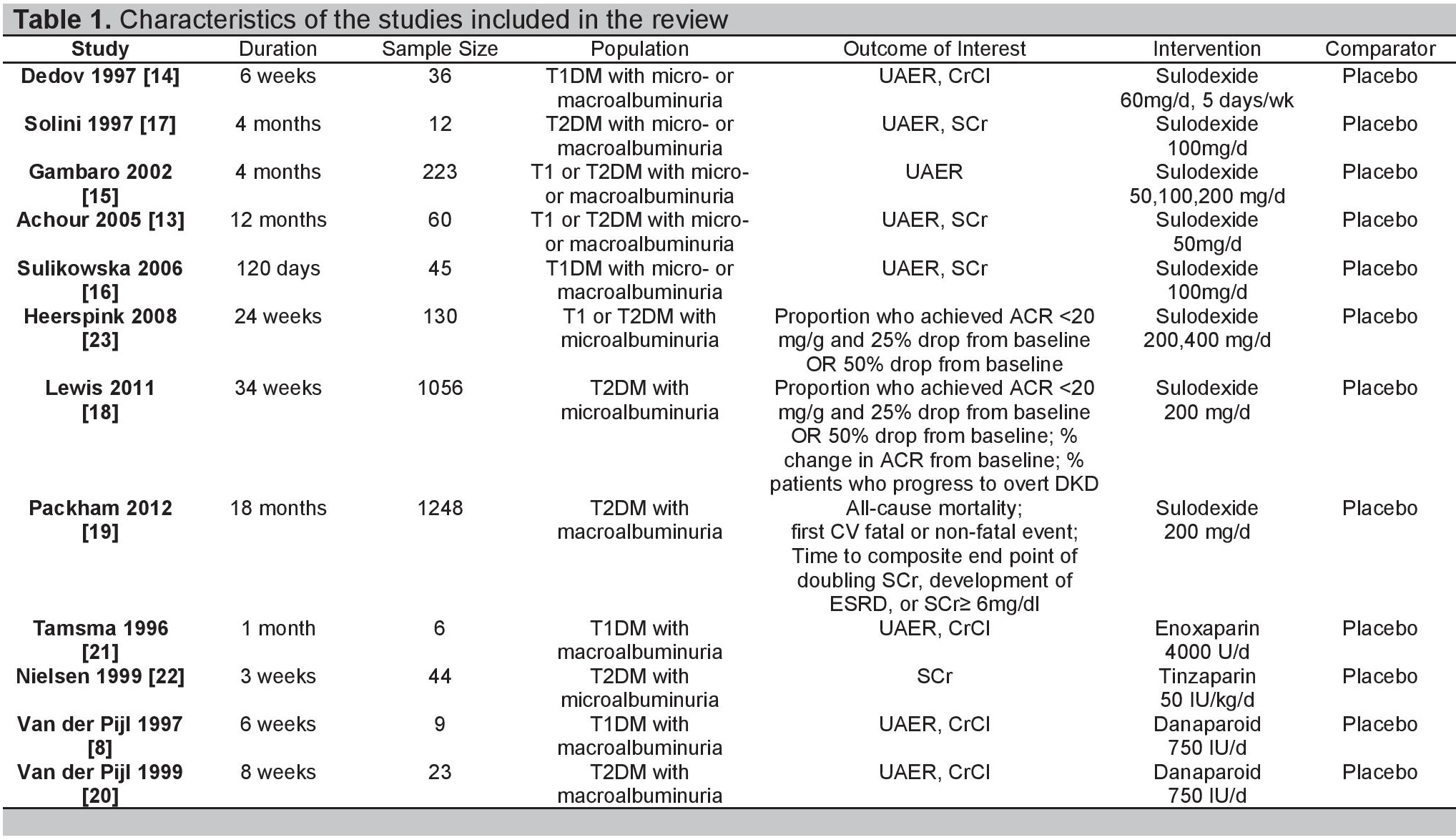
Table 1. Characteristics of the studies included in the review

Table 2. List of excluded studies and reasons for exclusion
Study Characteristics
The twelve included studies were all published from 1996- 2012 and totaled 2,892 patients. Most were conducted in Europe, with only three studies having participants outside of the region (Australia, New Zealand, Canada, USA, and Israel). Four studies dealt with Type 1 DM while five dealt with Type 2 DM; the remaining three included both types of patients. In terms of albuminuria, three studies involved microalbuminuria while four involved macroalbuminuria; the remaining five studies involved both stages of DKD. Eight had sulodexide as the main intervention with doses ranging from 50-400 mg/day (Gambaro et al., used three different doses while Heerspink et al., used two different doses) while two each had LMWH (enoxaparin and tinzaparin) and danaparoid as primary therapies. Treatment duration ranged from three weeks to 18 months and all studies had placebo as the comparator. Only one study evaluated all-cause mortality rate; the rest mostly evaluated changes in UAER and either SCr or CrCl, with additional endpoints being HbA1c, BP, and lipid levels, as well as titers of clotting parameters such as fibrinogen, von Willebrand factor, and antithrombin III. With regards to methodological quality, all studies were sufficiently randomized with adequate follow up rates. The baseline characteristics of the groups being compared also yielded no significant differences. Three studies were open-label while the rest were double-blind. However, allocation concealment was unclear in most of the studies; hence the overall methodological quality of the trials is at most moderate. Table 3 summarizes the risk of bias assessment for the included studies
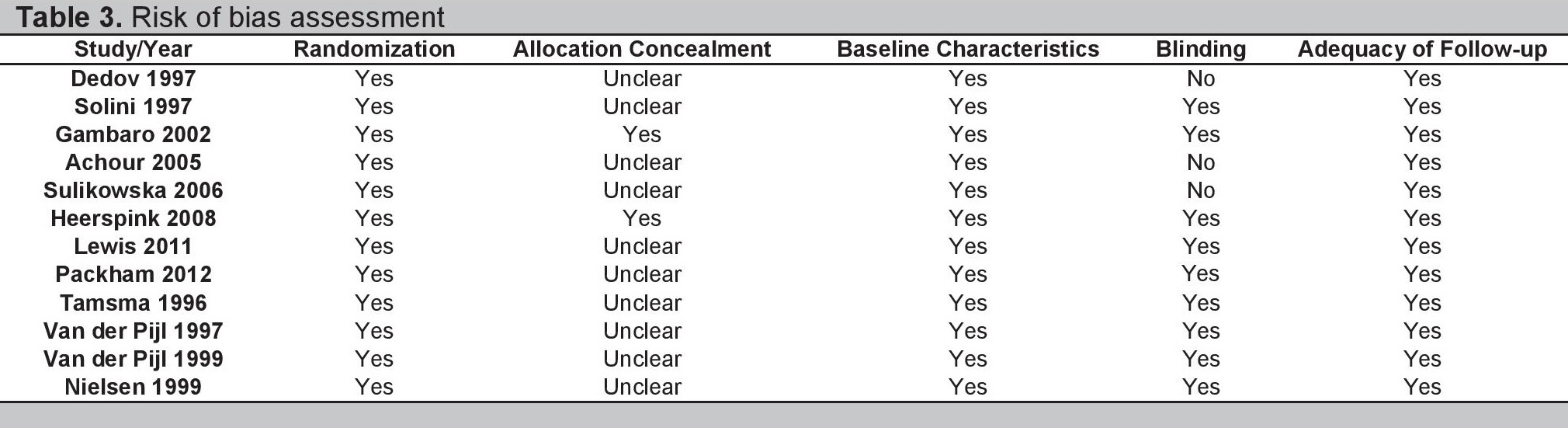
Table 3. Risk of bias assessment.
Data Synthesis
Effects of Heparinoid Supplementation on All-Cause Mortality
Only the study by Packham et al., evaluated all-cause mortality rate, which was not statistically significant between the sulodexide and placebo groups (95% CI, HR 0.79 [0.41, 1.53], p=0.49). The same study also found no statistically significant difference in the first cardiovascular fatal or non-fatal event between the two groups (95% CI, HR 1.12 [0.82, 1.54], p=0.48).[19]
Effects of Heparinoid Supplementation on Albuminuria
Five studies reported changes in UAER.[13],[15]-[17],[21] One used enoxaparin while the remaining four involved sulodexide. Gambaro et al., utilized three different sulodexide doses and was treated as three separate treatment arms (a, b, c). The analysis was carried out using the inverse variance method on log-transformed UAER values due to the skewed distribution of the sample. On initial analysis, no statistically significant difference (95% CI, log-transformed MD -0.78 mg/24h [-1.76, 0.21], p=0.12) was found between the heparinoid and placebo groups (Figure 2). Significant heterogeneity (I^2=57%) was present, hence the random effects model was used. To investigate the source of heterogeneity, sensitivity analysis was first performed by removing one study at a time, starting with the studies that had the highest potential risk for bias. We subsequently found no significant changes in the values of the pooled log-transformed MD, indicating the quality of the studies to be satisfactory. Post-hoc subgroup analysis was then performed according to type of DM. This time, acceptable homogeneity was seen for both subgroups (I^2=30% and I^2=0% for Type 1 and Type 2 DM, respectively). Moreover, a statistically significant decrease in UAER was found in the heparinoid group for Type 1 (95% CI, log-transformed MD -1.5 mg/24h [-2.79, -0.21], p=0.02) but not Type 2 DM (95% CI, log-transformed MD 0.13 mg/24h [-0.42, 0.68], p=0.65).
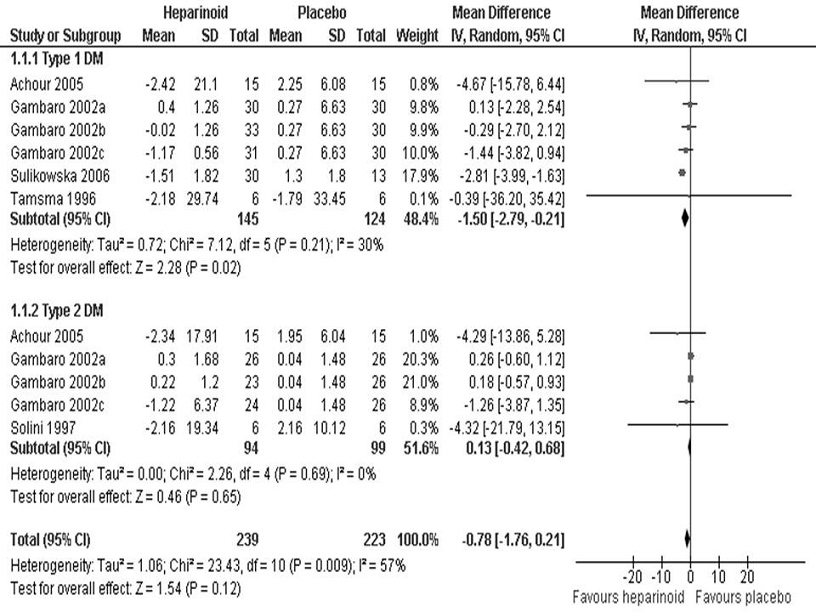
Figure 2. Log-transformed mean difference in urinary albumin excretion rates (mg/24 hours) between the heparinoid and placebo groups according to DM type.
Two trials also looked at the number of patients reaching therapeutic success, which we defined as either (1) an ACR <20 mg/g and at least a 25% drop from baseline; or (2) at least a 50% drop from baseline. This was similar to the criteria set in previous studies.[18],[23] Heerspink et al., utilized two different sulodexide doses and was treated as two separate treatment arms (a, b). For this analysis, the Mantel-Haenszel odds ratio was used since the variable was dichotomous. We found no statistically significant difference (95% CI, OR 0.97 [0.71, 1.33], p=0.87) in the patient number achieving therapeutic success between the heparinoid and placebo groups (Figure 3). We used the fixed-effects model since I^2 <y;50%.
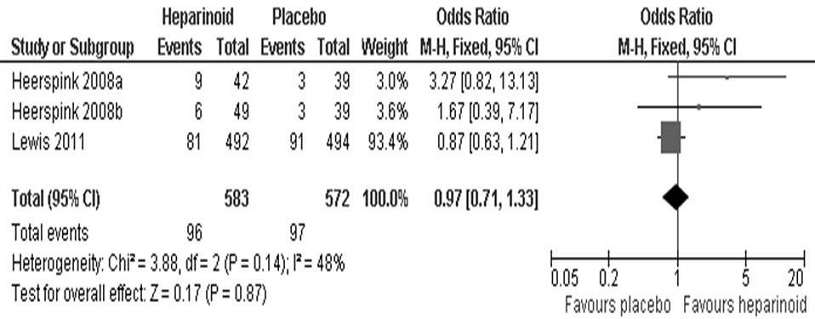
Figure 3. Mean difference in the proportion of patients attaining therapeutic success between the heparinoid and placebo groups.
Effect of Heparinoid Supplementation on Azotemia
Eight studies evaluated heparinoid effects on azotemia.8,14- 17,19-22 Three expressed the results in terms of SCr (Figure 4) while four expressed the results in terms of CrCl (Figure 5). The inverse variance method was used since the variables were continuous. We found no significant difference in both SCr (95% CI, MD 2.55 umol/L [-0.54, 5.65], p=0.11) and CrCl (95% CI, MD -8.55 mg/min [- 18.28, 1.18], p=0.09) between the heparinoid and placebo groups. The fixed effects model was used as no significant heterogeneity was seen for both SCr (I2=15%) and CrCl (I2=0%) analyses. The study by Packham et al., meanwhile, looked at a composite endpoint of SCr doubling, development of ESRD, or SCr ≥6 mg/dL and also did not find a statistically significant difference between the two groups (95% CI, HR 0.85 [0.50, 1.44], p=0.54).[19]
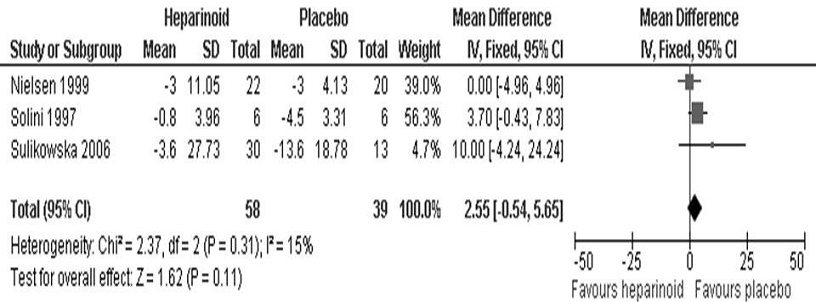
Figure 4. Mean difference in serum creatinine (umol/L) between the heparinoid and placebo groups.
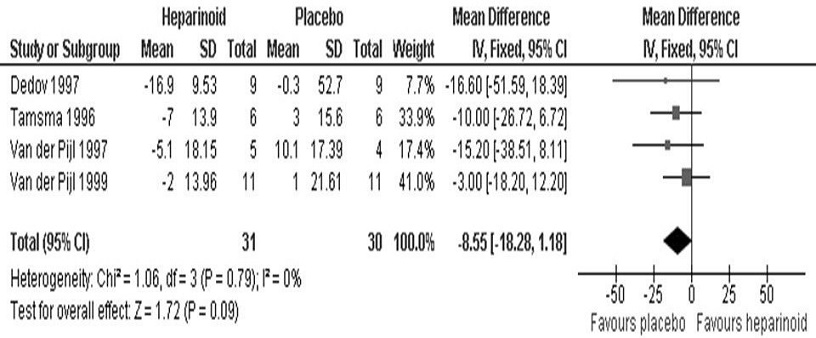
Figure 5. Mean difference in creatinine clearance (mg/min) between the heparinoid and placebo groups.
Adverse Effects
Nine studies briefly reported adverse events.[8],[13],[15],[18],[19]-[23] The incidence of likely study-related side effects was similar between the heparinoid (10.9% to 13%) and placebo (12.2% to 17.58%) groups. For the sulodexide studies, these included skin rash, nonspecific muscle aches, epigastric pain, diarrhea and a slight increase in liver function tests. For the LMWH and danaparoid studies, the most common complaint was a small, transient hematoma at the injection site. There were no significant changes in the levels of hemostatic variables and clotting parameters. Likewise, on ophthalmic evaluation, no progression of retinopathy or new onset hemorrhages were seen. Most importantly, no serious adverse event (SAE) was reported by any of the investigators as being possibly related to the study drug.
This review summarized the current available data on the efficacy of heparinoid supplementation in DKD patients. Compared to placebo, heparinoid-treated Type 1 DM patients experienced a statistically significant logtransformed MD of 1.5 mg/24h (or a raw MD of 31.62 mg/24h, p=0.02) in UAER. Given that the threshold of albuminuria is >30 mg/24h, this may be clinically significant. However, it is equally important to remember that the log-transformed lower limit of 0.21 mg/24h translates to a raw MD of only 1.62 mg/24h, which is clinically insignificant.
For heparinoid-treated Type 2 DM patients, we found a negligible log-transformed MD of 0.13 mg/24h (or a raw MD of 1.35 mg/24h, p=0.65) compared to placebo. While the result for Type 1 DM may affirm the renoprotective mechanisms of heparinoids, the lack of response for Type 2 DM supports the view of some articles that the nephropathy for both DM types may not be totally similar as was previously assumed. Micro- or macroalbuminuria may already be present when Type 2 DM is diagnosed, reflecting its long asymptomatic period; furthermore, hypertension more commonly accompanies DKD in Type 2 DM. Studies have also shown that glomerular changes are less pronounced in Type 2 DM, hence microalbuminuria may be less predictive of macroalbuminuria and progression to ESRD in these patients. Finally, it should be noted that the albuminuria in Type 2 DM may be secondary to other comorbidities including congestive heart failure, prostate disease, or concurrent infections. These different factors result in a heterogeneous pattern of renal disease and may explain the lesser predictability of response to therapy in Type 2 DM.[24]
For the number of patients reaching therapeutic success, the Mantel-Haenszel OR of 0.97 (p=0.87) implied no statistically significant difference between the two arms of the study, although this analysis was limited only to two studies on sulodexide. The reasons cited by these studies include the complex manufacturing requirements for sulodexide and the fact that having patients on preexisting maximal doses of an ACEI or ARB left little room for a significant superimposed heparinoid effect.[18],[23]
Also, we did not find statistically significant differences in all-cause mortality (HR 0.79, p=0.49) as well as in both SCr (MD 2.55 umol/L, p=0.11) and CrCl (MD -8.55 mg/min, p=0.09) between the heparinoid and placebo groups. This is consistent with data showing that the hypoalbuminuric effect of heparinoids appeared to be independent of any detectable variation in renal hemodynamics as reflected by SCr and CrCl.15 The rate of adverse events did not also differ significantly between the heparinoid and placebo groups, supporting the safety profile of the intervention.
This meta-analysis was limited by the generally small sample sizes of the trials (with two exceptions) and their intermediate methodological quality. The duration of treatment may have not also been long enough to sufficiently effect observable clinical changes. Moreover, there was a relative lack of data on other heparinoids, with two-thirds of the studies dealing with sulodexide alone. Data on hard endpoints was also scarce, with only one study evaluating all-cause mortality and only two studies evaluating achievement of therapeutic success.18,19,23 Similarly, no study evaluated other outcomes such as rates of dialysis and hospitalization and health-related quality of life. As the included trials were mostly conducted in Western countries, it may be important to see how heparinoid supplementation fares in DKD patients from other parts of the world. In Asia, for instance, DM patients have higher rates of microalbuminuria and faster progression to ESRD compared to their Western counterparts.[25]
Another important factor to consider is the baseline chronic kidney disease (CKD) stage of the subjects in the different studies. Save for the Packham study, whose subjects had a moderately decreased mean baseline estimated glomerular filtration rate (eGFR) of 31.4 ml/min (categorized as CKD Stage III), the rest of the trials included patients with relatively mild CKD (Stages I-II), regardless of DM type. This may have explained the lack of efficacy seen in the Packham study – resulting to its early termination – and somehow defeats the intended purpose of the intervention as an add-on drug for advanced CKD. Additional sensitivity analyses were not warranted as the Packham study was not included in any of the forest plots.
Since no outcome contained more than ten studies, we agreed not to do a funnel plot as it can be misleading.26 An attempt to minimize publication bias was done instead by extensively searching for unpublished data, while minimization of selection bias was done via pre-specified inclusion and exclusion criteria, performance of a systematic search, and independent evaluation of trial quality by two reviewers. Although the results of this study suggest a beneficial hypoalbuminuric effect of heparinoids in Type 1 DM patients, data remains limited to warrant routine clinical use. Larger trials are needed to further evaluate their application in DKD.
Heparinoid supplementation was not associated with statistically significant changes in all-cause mortality, SCr, CrCl, and achievement of therapeutic success for both Type 1 and Type 2 DM patients. However, it may be associated with a statistically significant UAER reduction of approximately 31.62 mg/24h as compared to placebo in Type 1 DM patients. Due to lack of data on hard endpoints as well as optimal dosing and duration of therapy, we cannot yet recommend its routine use for DKD patients. More studies involving larger populations are recommended.
Statement of AuthorshipAll authors certified fulfillment of ICMJE authorship criteria.
Author DisclosureThe authors have declared no conflict of interest.
Funding SourceNone.
[1] Ayodele OE, Alebiosu CO, Salako BL. Diabetic nephropathy--A review of the natural history, burden, risk factors and treatment. J Natl Med Assoc. 2004;96(11):1445-54. PubMed PMCID.