The World Health Organisation (WHO) has estimated a rise in the prevalence of diabetes from 4.7% to 8.5% in the adult population worldwide between 1980 and 2016. This rise in prevalence has been faster in low- and middle-income countries than in high-income countries.[1] Diabetes mellitus is a common medical complication of pregnancy. Gestational diabetes mellitus (GDM) is defined as any glucose intolerance with onset or first recognition during pregnancy.[2] One in 25 pregnancies is affected worldwide and about 4 million women have GDM in India.[3] The variation in the prevalence is due to the difference in race, ethnicity, age, body composition and screening and diagnostic criteria used in that particular population.
The increasing trend of prevalence of diabetes and GDM is due to the rising incidence of obesity and changing lifestyle patterns. Women with GDM are at a higher risk of both maternal complications like gestational hypertension, pre-eclampsia, operative delivery and fetal complications like macrosomia, birth injuries, stillbirths, neonatal hypoglycemia and hyperbilirubinemia. In addition, these women and their offsprings are known to develop type 2 diabetes mellitus later in life.[1]
The screening and diagnosis of GDM has been a matter of considerable debate. The first diagnostic criteria of GDM was proposed by O’Sullivan using 100 g, 3-hour oral glucose tolerance tests (OGTT) which were modified by Carpenter and Coustan in 1982.[4],[5] The International Association of Diabetes and Pregnancy Study Group Consensus Panel (IADPSG) recommended a one-step 75 g OGTT based on results of the Hyperglycemia and Adverse Perinatal Outcomes (HAPO) study.[6],[7] The WHO has also recommended this strategy since 2013.[8] The American Diabetes Association (ADA) adopted the IADPSG recommendation since 2010, but emphasised in 2014 that a one-step 75 g OGTT or two-step approach with a 50g oral glucose challenge test (OCT) followed by a 3-hour 100 g OGTT at 24-28 weeks of gestation for those who screen positive, are both equally efficacious in diagnosing GDM, pending long-term outcome studies.[9] However, American College of Obstetricians and Gynecologists (ACOG) continues to recommend the two-step strategy for diagnosis of GDM.[2]
This study was undertaken to compare the prevalence of GDM and feto-maternal outcomes between CC and IADPSG groups.
METHODOLOGYA cross-sectional comparative analysis of women who were screened and diagnosed GDM at a tertiary care teaching hospital was performed. The protocol for screening and diagnosis of GDM at our hospital was changed from that of a two-step approach using Carpenter & Coustan criteria to a one-step approach using the IADPSG criteria in 2011. Data of women screened and diagnosed as GDM between April 2006-March 2007 (using Carpenter & Coustan criteria) and April 2013-March 2014 (using the IADPSG criteria) were included in the study. The study protocol was approved by the Institute’s Ethics Committee. Waiver of consent was granted as it was a retrospective study and patient confidentiality was maintained.
In the CC group, women who screened positive at 24–28 weeks of gestation (plasma glucose of ≥140 mg/dL after 1 hr of a 50 g GCT), underwent a 3 hr 100 g oral glucose tolerance test (OGTT) and were diagnosed GDM when at least two values were more than or equal to the following threshold values: Fasting plasma glucose-5.3 mmol/L; 1 hr-10.0 mmol/L; 2 hr-8.6 mmol/L; 3 hr-7.8 mmol/L. In the IADPSG group, women underwent a single-step 75 g OGTT and were diagnosed GDM when one or more of the following threshold values were exceeded: Fasting plasma glucose 5.1 mmol/L;1 hr-10 mmol/L; 2 hr-8.5 mmol/L.
The antenatal care protocol followed remained the same for both the cohorts. Women with GDM were initially treated with diabetic diet for 1-2 weeks and plasma glucose was measured in the fasting and post-meal states. When the target plasma glucose values (fasting 5.0 mmol/L and post-meals 6.7 mmol/L) were exceeded even with diabetic diet, insulin was started and dose titrated to achieve the target plasma glucose values.
Maternal and perinatal outcomes in both these groups were noted from the case files of these women. The maternal outcomes studied were polyhydramnios, hypertensive disorders in pregnancy, mode of delivery and shoulder dystocia. Neonatal outcomes studied were birth weight, respiratory distress syndrome and hyperbilirubinemia. These outcomes were compared between the two groups.
Data were entered in Microsoft Office Excel spreadsheet and analysed using SPSS software version 19.0. Means and proportions were calculated for continuous and categorical variables respectively. Continuous data were tested for normality using the Shapiro-Wilk test. Normally distributed data were presented as mean ± SD and analysed using t-test. Data that were not normally distributed were presented as medians and analysed using Mann-Whitney U test. Pearson Chi-square test was used to find the association between the various outcomes between the two groups. A p-value <0.05 was considered as statistically significant.
RESULTSFive hundred pregnant women were screened between April 2006-March 2007 and 36 were diagnosed GDM using Carpenter & Coustan criteria (CC group). After the protocol for screening and diagnosis of GDM at the hospital was changed from a two-step to a one-step approach, 733 women were screened between April 2013-March 2014. Among them, 167 women were diagnosed as GDM using the IADPSG criteria (IADPSG group). The prevalence of GDM was 7.2% in the CC group and 22.78% in the IADPSG group (p=0.000).
Majority of women in both groups were primigravidae (54% and 43.7% in CC and IADPSG groups respectively). Median age of women in the CC group was 25 years (interquartile range 23.50-27.00) and 26 years (interquartile range 23.00-30.00) in the IADPSG group. There was a statistically significant difference in the number of women who developed hypertension and polyhydramnios among the two groups (Table 1). Women who had an operative vaginal delivery (16.67% vs.6.6%, p=0.085) were higher in the CC group than the IADPSG group and mean birth weight (3.10 ± 0.55 kg vs. 2.97 ± 0.48 kg, p=0.165) was higher in the CC group than the IADPSG group. However, both these outcomes were not found to be statistically significant. Among the perinatal outcomes, a statistically significant improvement was found in the number of neonates developing respiratory distress syndrome (p=0.00) and hyperbilirubinemia (p=0.00) in the IADPSG group.
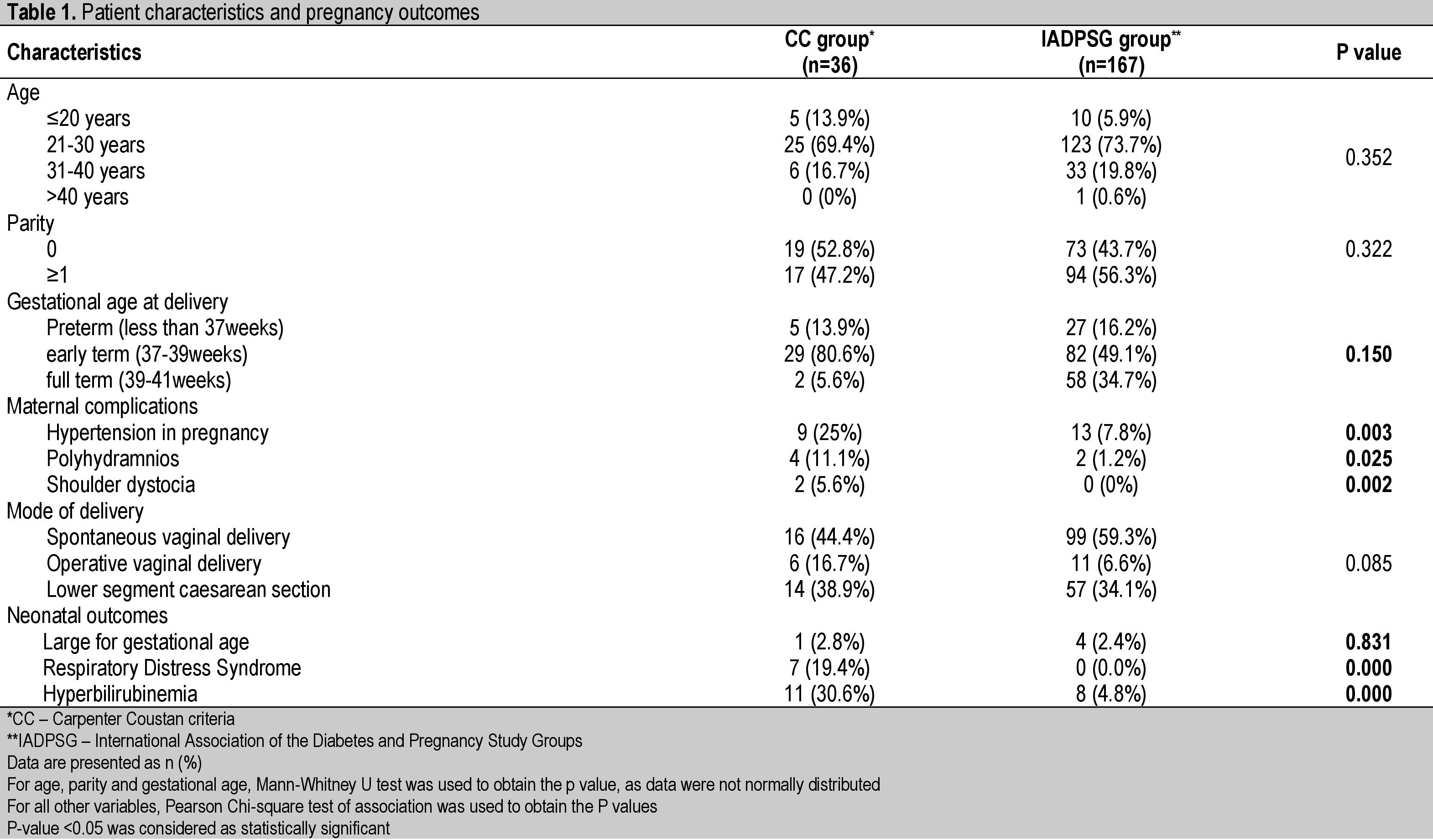
Table 1. Patient characteristics and pregnancy outcomes
The prevalence of GDM was found to have increased from 7.2% to 22.78% when diagnostic criteria were changed from CC criteria to the IADPSG criteria. This increase in prevalence was similar to that observed by many other authors (Table 2).[10],[11],[12],[13],[14] The prevalence was found to be as high as 35.5% in one study conducted among a Spanish population.13 This is mainly due to the lower threshold values for diagnosis as per the IADPSG criteria and noted originally in the HAPO study as well.
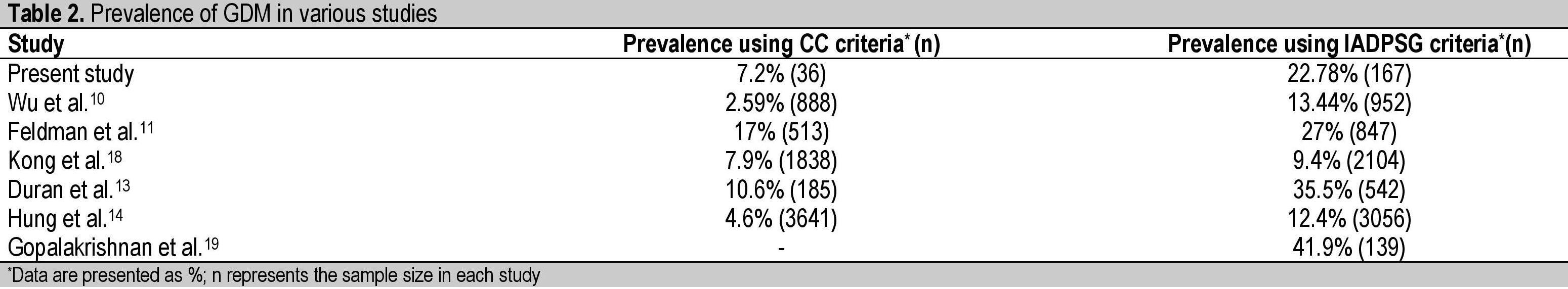
Table 2. Prevalence of GDM in various studies
The rate of preterm deliveries was not significantly different among the two groups, like that reported among Belgian, Spanish and Taiwanese cohorts.[13]-[15] The rates of hypertension, polyhydramnios and mean birth weights were found to have decreased after changing the diagnostic protocol to the IADPSG criteria. However, only hypertensive disorders in pregnancy, polyhydramnios and shoulder dystocia were significantly reduced in the IADPSG group in our study (Table 1). This appears to be due to a higher number of women being treated for gestational diabetes. Similarly, Duran et al., in their study found a statistically significant decrease in maternal hypertension and large for gestational age (LGA).[13] Hung et al., also demonstrated an improvement in perinatal outcomes like LGA and caesarean delivery rates.[14] This is in contrast with the results obtained by other authors who did not find any significant changes in most other outcomes (Table 3).
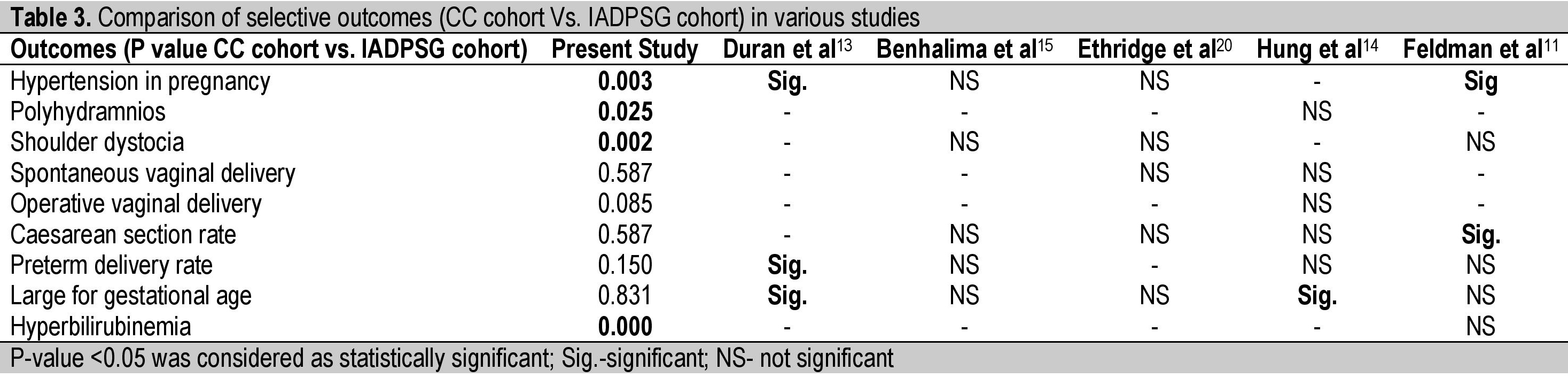
Table 3. Comparison of selective outcomes (CC cohort Vs. IADPSG cohort) in various studies
A major limitation of our study is its retrospective nature and another is the small sample size. Data on the maternal weight gain during pregnancy, the pre-pregnancy BMI, high risk factors like prior GDM and blood sugar control are lacking. Further, the two criteria were applied on different groups of women and in different time periods, which could have influenced the prevalence in the IADPSG group due to a general background increase in prevalence of obesity and type 2 DM in the population.
The results of the HAPO study[6] demonstrated an increasing risk of adverse maternal, fetal and neonatal outcomes with increasing maternal glycemic levels. These glycemic levels were observed to be well within the normal range as per the previously followed diagnostic cut-offs for diagnosis of GDM. Based on these findings, although the IADPSG and WHO adopted these criteria in 2010 and 2013 respectively, FIGO advocated the use of these criteria only in 2015. However, considering the limitations in medium to low resource countries, FIGO still considers alternative strategies like the one step non-fasting 75 g OGTT as recommended by the Diabetes in Pregnancy Study Group in India (DIPSI), as equally acceptable for diagnosis of GDM. In the DIPSI test, a glucose level of ≤7.8 mmol/L or ≤140 mg/dL is taken as the cut-off for diagnosis of GDM.[16],[17]
Following alternative strategies like the DIPSI test makes the comparison in outcomes more complex and hence it would be more useful to evaluate outcomes using IADPSG criteria alone and comparing these with the non-diabetic population as determined by these criteria. From the results of our study, important maternal and neonatal outcomes were found to be statistically significantly better when the newer IADPSG criteria are applied for diagnosis. This may have been due to lower threshold values used for diagnosis and consequently earlier intervention and treatment in the IADPSG group. Our study is not adequately powered to determine if this change is truly significant. As most national and international organisations seem to be accepting and adopting the IADPSG criteria, large multicentre population and hospital based studies are needed to demonstrate a significant improvement in the pregnancy outcomes using these criteria.
The diagnosis of Gestational Diabetes Mellitus (GDM) is presently being made by different criteria applied to various populations and with different approaches, taking into consideration the health services, resources and prevalence of glucose intolerance in the population. FIGO now advocates universal screening of all pregnant women for GDM by the single-step 75 g OGTT using IADPSG criteria. The use of alternative strategies in some countries is still acceptable, but causes confusion and inability to draw adequate comparisons between the different outcomes. Our study demonstrated a significant improvement in important maternal and perinatal outcomes, but is limited by its small sample size. Further large prospective trials are required to validate these results.
Statement of AuthorshipAll authors certified fulfillment of ICMJE authorship criteria.
Author DisclosureThe authors have declared no conlict of interest.
Funding SourceNone.
[1] Roglic G. WHO Global report on diabetes: A summary. Int J Non-Commun Dis. 2016;1(1):3-8. Available from: http://www.ijncd.org/text.asp?2016/1/1/3/184853.