Acromegaly is characterized by somatic overgrowth, physical disfigurement, multiple co-morbidities and premature death. The main cause of this disorder is chronic hypersecretion of GH, mediating its effects directly or through IGF-1.
The control of GH secretion is the most important determinant in reducing mortality in acromegaly. Any therapy that is able to reduce GH and IGF-1 will alter the prognosis of a patient with acromegaly. Octreotide is able to normalize IGF-1 in 68% of patients and reduce GH to less than 5 μg/L in 53%.[3] A multi-center prospective study demonstrated tumor shrinkage with octreotide in all 27 newly diagnosed patients with acromegaly, with median tumor volume reduction of 49% in microadenomas and 43% in macroadenomas.[4] Thyroid cancer may also be an associated finding in long-standing undiagnosed acromegaly, as illustrated in this case report.
CASEA 58-year-old Malay lady with underlying type 2 diabetes and hypertension for 2 years was admitted to a nearby hospital for lacunar stroke. During assessment, she was noted to have features of acromegaly. On further interview, she disclosed increased shoe (from 6 to 11) and ring (14 to 26) sizes. She also experienced hoarseness, increased sweating, weight gain and higher blood pressure on monitoring. She also observed an enlarging goiter but denied obstructive symptoms. She had severe sleep apnea symptoms based on the Epworth Sleepiness scale. There were no known endocrine disorders or malignancy in her family.
Physical examination revealed marked features of acromegaly: frontal bossing, prognathism, increased interdental separation, thick lips and coarsened facial features. Her blood pressure was 160/90 mmHg, with no clinical evidence of heart failure. She had a hard goiter with palpable cervical lymph nodes. She had moist palms and skin tags. Visual field was intact on confrontation test.
Initial tests showed elevated IGF-1 (703 μg/L, reference range 35-210) and fasting GH (10.3 ng/mL, reference range up to 8). GH was not suppressed by oral glucose loading, with a nadir of 12.9 μg/L. Follicle stimulating hormone (26.5 IU/L) and luteinizing hormone (8.6 IU/L) were low for age. Other parameters were within reference ranges (prolactin 150 IU/mL, thyroid stimulating hormone 1.15 µIU/mL, free thyroxine 13.8 pmol/L, corrected Ca 2.36 mmol/L, serum Na 138 mmol/L, serum K 4.0 mmol/L and serum creatinine 44.9 µmol/L). HbA1c was 6.9%. Sleep study yielded an Apnea-Hypopnea Index (AHI) of 36.5, indicative of severe OSA. Echocardiogram performed in preparation for eventual surgery showed concentric LVH and diastolic dysfunction with an ejection fraction of 65%. Magnetic resonance imaging (MRI) of the pituitary revealed a 1.5 cm x 1.8 cm x 1.1 cm macroadenoma located at the anterior pituitary invading the right cavernous sinus and encasing the right internal carotid artery. There was a clear plane separating the pituitary from the optic chiasm (Figure 1A).
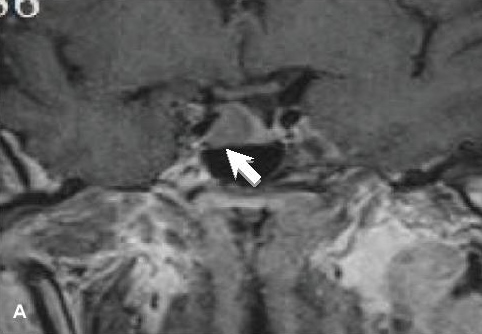
Figure 1a. MRI of the pituitary gland, coronal view. A, T1-weighted initial study showed a 1.5 cm x 1.6 cm x 1.1 cm adenoma (white arrow) on the right side of the pituitary invading the right cavernous sinus and encasing the right internal carotid artery.
Neck ultrasonography showed multiple mixed solid and cystic nodules in both thyroid lobes. The thyroid nodule on the right lobe appeared solid and cystic, with wall calcification and no vascularity (Figure 2A). There were multiple predominantly solid thyroid nodules with internal microcalcifications on the left lobe, with the largest nodule measuring 3.2 cm x 3.2 cm x 4.6 cm located at the lower pole (Figure 2B). Subcentimeter cervical lymph nodes were also seen. Fine needle aspiration cytology of the thyroid nodule reported neoplastic thyroid epithelial cells displaying enlarged nuclei, nuclear grooves and intranuclei inclusions suggestive of papillary thyroid cancer.
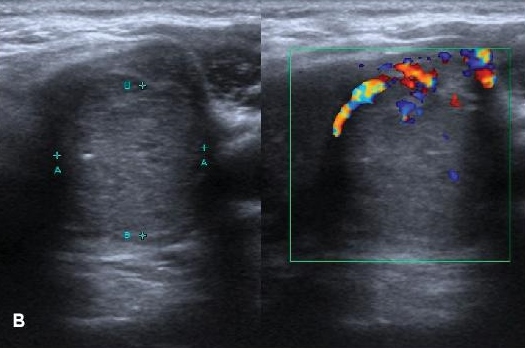
Figure 2b. Thyroid ultrasonography. B, predominantly solid nodule with internal microcalcifications measuring 3.2 cm x 3.2 cm x 4.6 cm at the lower pole of the left lobe.
Medical therapy with subcutaneous octreotide LAR 20 mg monthly for 6 months prior to surgery was initiated to alleviate cardiovascular, metabolic and respiratory co-morbidities. Recent lacunar strokes were also a contraindication to immediate surgery. After 3 months of medical therapy, clinical features of acromegaly and OSA improved, and IGF-1 became normal.
She underwent total thyroidectomy with therapeutic anterior and left lateral neck dissection. Histopathologic evaluation revealed papillary thyroid cancer measuring 5 cm x 5 cm, with involvement of 5 of the 8 resected lymph nodes. Subsequent remnant ablation with radioactive iodine 120 mCi was done. Post-treatment whole body scan (WBS) showed uptake at the area of the thyroid bed. Follow-up MRI after 6 months of octreotide LAR revealed a residual 0.5 cm x 0.6 cm x 0.3 cm pituitary microadenoma (Figure 1B). Transphenoidal adenomectomy was uneventful, with no post-operative pituitary hormonal deficit and diabetes insipidus. IGF-1 remained within normal range for age and gender.
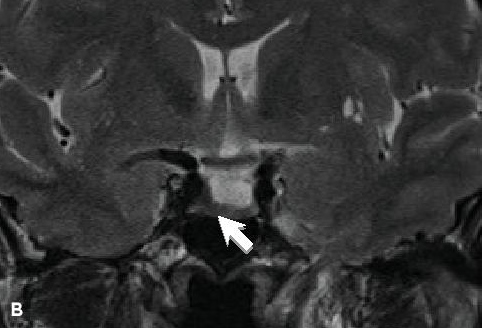
Figure 1b. MRI of the pituitary gland, coronal view. B, on T2-weighted imaging, the tumor appeared hypointense, suggestive of dense granulation.
The insidiousness of acromegaly often results in a late diagnosis, as seen in our patient. High GH and IGF-1 levels affect many tissues, causing cardio-metabolic consequences and respiratory complications mainly due to central and obstructive sleep apnea. Our patient presented with difficult to control blood pressure, stroke and severe OSA.
Treatment choices in acromegaly are dependent on several factors. In our patient, the macroadenoma was confined to the sella, with apparent invasion of the right cavernous sinus and encasement of the right internal carotid artery. As it was not near the optic chiasm, there were no visual and neurologic disturbances. Urgent surgical removal was not deemed necessary at that point.
SRL pre-treatment is not routinely recommended for acromegaly with macroadenoma.[5] However, a somatostatin receptor ligand (SRL) or GH receptor antagonist such as pegvisomant may be used as initial adjuvant medical therapy in a patient with significant disease (moderate to severe signs and symptoms of GH excess without local mass effects) to improve cure rate. Pre-operative long-acting octreotide treatment in patients with invasive pituitary macroadenomas for 6 months improved metabolic, hemodynamic parameters and reduced duration of hospital stay.[6] By reducing GH hypersecretion, SRLs are efficacious in decreasing tissue overgrowth in the heart and airways, thereby alleviating respiratory and cardiovascular co-morbidities. These were evident in our patient, as seen in the reduction of her IGF-1 and blood sugar levels, improvement in blood pressure and cardiac function, and reduction of severity of OSA leading to eventual discontinuation of continuous positive airway pressure. Tumor size was reduced by 67%, compared to approximately 50% reported in literature (Figure 1C). This reduction in size was accompanied by regression of cavernous sinus invasion, which improved surgical success.
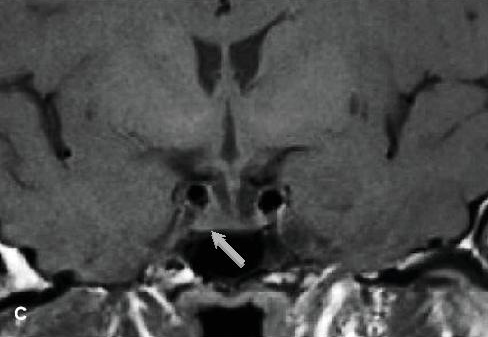
Figure 1c. MRI of the pituitary gland, coronal view. C, follow-up study showed reduction in size of the pituitary adenoma to 0.5 cm x 0.6 cm x 0.3 cm (thin gray arrow) after 6 months of octreotide LAR.
Response to SRL is said to be dependent on tumor consistency and the amount of somatostatin receptor subtype 2 (sst2) expression. Tumors that are densely granulated respond better compared to fibrous ones. Hypointense tumor signal on T2-weighted MRI, which correlate with dense tumor granularity, portend a favorable SRL response, as observed in the patient (Figure 1B). These factors can be used to guide the choice of pharmacotherapy in treating acromegaly.
The control of IGF-1 levels may mitigate the signs, symptoms and complications of acromegaly. Nadir GH is the most important determinant of mortality, followed by blood pressure and heart disease.[7],[8],[9] Symptom duration and diabetes seem to be less important determinants. Life expectancy becomes similar to the general population when GH levels are under control.[7],[8] Normalization of IGF-1 is also one of the goals of treatment in acromegaly. In a study of 466 acromegaly patients due to pituitary macroadenoma, SRL was able to normalize IGF-1 in 39.9%.[9] This is mainly due to the downregulation of GH release through the sst2 receptor.
One of the main setbacks of pituitary surgery is the occurrence of post-operative pituitary hormonal failure. In an analysis of complications of transsphenoidal surgery, anterior pituitary insufficiency occurred in 19.4% of patients.1 This risk, along with limited access to highly competent surgeons, have created a strong interest in medical therapy in acromegaly, with the goal of removing the risk of pituitary failure brought about by surgery.
Fifteen percent of mortality in acromegalic patients are attributed to malignancy. High levels of IGF-1 and IGF-binding protein 3 (IGFBP3) are seen in acromegaly. Activation of IGF-1 receptors causes cell proliferation and growth, whereas IGFBP3 promotes apoptosis. By inducing increased IGF-1 and IGFBP3 levels, excess GH promotes dysregulated cell growth characterized by dynamic signals for cell apoptosis as against cell growth. High GH leads to tissue growth, including the thyroid gland. Thyroid nodules are seen more frequently in acromegaly. Multinodularity and size correlates with duration of high IGF-1.[10] Increased tissue growth suggests malignant transformation of the thyroid nodules, as seen in our patient. Thyroid cancer was found to be the most common type of malignancy in acromegaly.[11] Our case report underscores the importance of routine screening by ultrasonography of the thyroid in these patients.
With a tumor size of more than 5 cm, capsular invasion and involvement of central and ipsilateral lymph nodes, our patient had stage IV papillary thyroid cancer (T4aN1bM0) based on the American Joint Committee on Cancer (AJCC) classification. Treatment involved total thyroidectomy with central and left lateral lymph node resection plus remnant ablation with 100 to 200 mCi radioiodine (with post-treatment WBS).[12] Follow-up of papillary thyroid cancer includes monitoring for tumor recurrence in lymph nodes, measurement of thyroglobulin and regular neck ultrasound. Monitoring of differentiated thyroid cancer has moved away from regular WBS following the availability of very sensitive assays for detection of thyroglobulin and high quality neck ultrasonography.
Our case demonstrated the successful pre-operative use of octreotide LAR for 6 months, which resulted to remission of acromegaly without any pituitary hormonal failure. While pre-treatment with SRL is not generally recommended at this time, it was able to reduce tumor volume more than what has been commonly reported, and also improved our patient’s outcome.[13] Our case also emphasized the need for close evaluation for thyroid malignancy, aside from surveillance of colonic polyps and cancer. While surgery is the current main treatment of acromegaly, the use of medical therapy, particularly SRL, may achieve remission and improve survival rate. The role of medical therapy in acromegaly for IGF-1 normalization to control concomitant malignancy warrants further research and insight.
Ethical ConsiderationPatient consent was obtained before submission of the manuscript.
Statement of AuthorshipAll authors certified fulfillment of ICMJE authorship criteria.
Author DisclosureThe authors declared no conflict of interest.
Funding SourceNone.
[1] Colao A, Ferone D, Marzullo P, Lombardi G. Systemic complications of acromegaly: Epidemiology, pathogenesis, and management. Endocr Rev. 2004;25(1):102-52. PubMed DOI.