The World Health Organization (WHO) estimates that 41 million children under 5 years old worldwide are obese, with 48% dwelling in Asia.1 The numbers are rapidly increasing even in developing countries, especially in urban areas. In Malaysia, the National Health and Morbidity Survey (NHMS) showed an alarming surge in the proportion of children less than 18 years who are obese from 3.9% (0.3 million) in 2011 to 11.9% (1 million) in 2015.2 Without prompt intervention, childhood obesity will continue into adulthood, leading to the development of complications which increase morbidity and mortality, reduce life expectancy and impair quality of life.[1],[2],[3] Metabolic syndrome is a constellation of metabolic derangements consisting of abdominal obesity, dyslipidemia, hypertension and raised plasma glucose that significantly increases the risk of developing type 2 diabetes and premature atherosclerotic cardiovascular disease.[4],[5] The prevalence of pediatric metabolic syndrome is also increasing in obese adolescents, with rates as high as 28.7% in the United States National Health and Nutrition Examination Survey 1988-1994.[3] A single unified definition for pediatric metabolic syndrome was adopted only in 2007 based on the International Diabetes Federation Consensus Definition.6 Early recognition is vital to enable prompt institution of early childhood lifestyle intervention and to prevent progression and development of complications.[6],[7] When similar criteria were applied to a wider age group of children aged 4 to 20 years, the prevalence of metabolic syndrome in the study population was 38.7% in moderately obese children (BMI >95th percentile), compared to 49.7% in severely obese (BMI >98th percentile).[8] An increase in the individual components of metabolic syndrome, particularly high plasma glucose, triglycerides and systolic blood pressure, and low HDL, were found together with increasing BMI. It can be surmised that the prevalence of metabolic syndrome increased with increasing obesity, and was higher in obese compared to overweight subjects. A thorough history and clinical examination to locate clues for the identification of children at risk of developing metabolic complications of childhood obesity stemming from insulin resistance is essential. This includes assessment of visceral adiposity in view of its association with increased metabolic risk. Clinicians are reminded to maintain a high index of suspicion for clinically silent conditions, such as impaired glucose tolerance and non-alcoholic fatty liver disease, which may be present in this group of high risk children.[9]
Several researchers have investigated the prevalence of metabolic syndrome in the pediatric population in Malaysia. In a 2010 study on 78 schoolchildren aged 8 to 10 years old in Kuala Lumpur, 17.9% of participants were found to be obese, of which 2.9% were assessed to have the metabolic syndrome based on IDF criteria.[10] The relatively low prevalence of metabolic syndrome even in obese subjects in comparison to foreign studies was attributed to the high cut-off values used by the IDF criteria. Upon analysis of normal weight children, 9.1% had one unspecified risk factor for metabolic syndrome.[10] The study was limited by the lack of local population-specific waist circumference percentile charts at the time it was carried out, and the relatively narrow age range of participants.
In a similar study, Wee and colleagues examined 402 participants aged 9 to 12 years in Kuala Lumpur for the risk factors of metabolic syndrome. The prevalence of overweight/obesity was 5.3%, of which 5.3% had metabolic syndrome by IDF 2005 criteria.[11] At least one risk factor for metabolic syndrome was identified in 88% of obese participants, compared to 14% in those with normal weight. Comparison between overweight and obese subjects was not carried out in this study. The prevalence of metabolic syndrome was determined only in overweight/obese participants. This study was also unable to refer to age- and gender-specific local waist circumference standards. The authors attributed the lack of association between age, gender and ethnicity with metabolic syndrome to the small sample size.[11]
A recent study conducted in 2014 on 1,014 schoolchildren found that 16% were overweight and 9.4% were obese. The prevalence of metabolic syndrome by IDF criteria was 2.6% in the general study population, and 10% in the overweight/obese participants. The risk factors for metabolic syndrome were predominantly clustered in the overweight/obese group, compared to the normal weight subjects.[12] The study was limited by the inclusion of children aged 13 years old only. A comparison of risk factors between overweight and obese children was also not included.
Body mass index is a less sensitive indicator of abdominal adiposity in comparison with waist circumference, as it does not account for body fat distribution. Children with lower BMI but with greater waist circumference are at higher risk of having metabolic syndrome compared to those with higher BMI but lower waist circumference. This study aims to compare the clinical and biochemical profile of metabolic syndrome in obese children less than and more than 10 years of age attending Paediatric clinic HUSM. The cut-off point of age 10 years was chosen as it is the average age of onset of puberty in both sexes in lieu of formal pubertal assessment in the form of Tanner staging.[13],[14] Puberty is associated with increased insulin resistance, which is the primary pathophysiologic mechanism for the development of metabolic syndrome. Therefore, it is hoped that this study will be able to demonstrate if there is any significant difference in the outcomes which predict predisposition to metabolic syndrome between these two groups of obese children.
Study Procedure
Patients under 18 years old who had previously been followed up at Paediatric clinic HUSM for overweight or obesity over a 10-year period from 2006 to 2015 were identified with the assistance of the HUSM record office. Clinical records were reviewed to obtain the demographic, anthropometric, clinical and biochemical parameters. Subjects who did not fulfill the inclusion criteria and those with incomplete data were excluded.
Inclusion and exclusion criteriaChildren less than 18 years old on Paediatric clinic HUSM follow up for obesity (BMI >95th percentile for age and sex based on CDC growth charts) between 2006 to 2015 were included. Patients with underlying history of medication use or with conditions affecting body fat composition/distribution, such as Cushing’s syndrome, hypothyroidism and Prader-Willi syndrome, were excluded. Children on medications that may alter blood glucose, lipid metabolism or blood pressure were also not included.
Statistical AnalysisData analysis was performed using SPSS (IBM) version 22. Descriptive statistics were used to summarize the socio-demographic characteristics of subjects. Numerical data were presented as mean (SD) or median (IQR) based on their normality distribution. Categorical data were presented as frequency (percentage). Means were compared with either independent T-test or Mann-Whitney depending on data distribution. For association between age and clinical/biochemical parameters, Chi-square test was used followed by multivariable analysis with logistic regression. Spearman rank was used to determine any relationship between two numerical variables as the data was not normally distributed.
Operational Definitions- Overweight: BMI >85th percentile and <95th percentile for age and sex according to Centers for Disease Control and Prevention (CDC) growth chart
- Obesity: BMI at or >95th percentile for age and sex according to CDC growth chart
- Hypertension: SBP >130 mmHg and/or DBP >85 mmHg
- Diabetes: FPG >6.9 mmol/L or random plasma glucose >11.1 mmol/L
- Impaired glucose tolerance: plasma glucose level 2 hours after a 1.75 g/kg (maximum 75 g) oral glucose challenge 7.77 to 11.1 mmol/L
- Impaired fasting glucose: FPG 5.6 to 6.9 mmol/L
- Abdominal obesity: waist circumference (WC) >90th percentile
- Metabolic syndrome: abdominal obesity with 2 or more of the following:
- Serum TG >1.7 mmol/L
- HDL <1.03 mmol/L
- SBP >130 mmHg or DBP >85 mmHg
- FPG >5.6 mmol/L
The study was approved by the Human Research Ethics Committee (HREC) USM (JEPeM code: USM/JEPeM/16040165).
A total of 101 subjects with follow up at Paediatric clinic HUSM for obesity from 2006 to 2015 were identified. Only 84 of the patient records retrieved were eligible for the study. The subjects were divided into 2 groups: group 1 consisted of obese children below 10 years of age, while group 2 included those 10 years and older. The demographic characteristics of both groups are illustrated in Table 1.
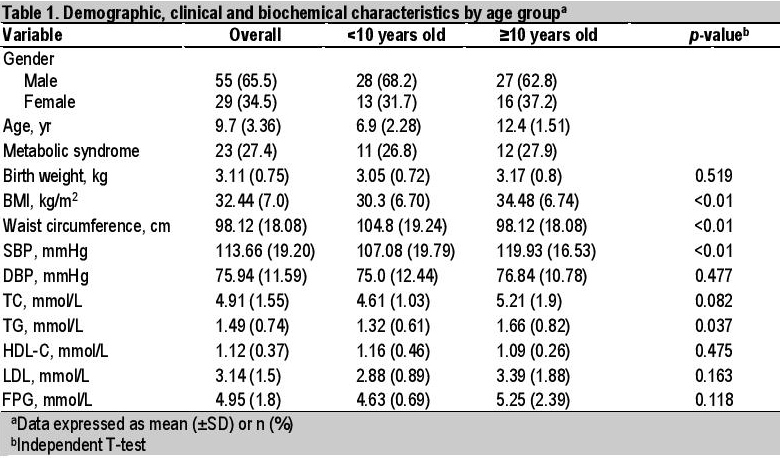
Table 1. Demographic, clinical and biochemical characteristics by age group
Majority of the 84 available subjects were boys, accounting for 68.2% and 62.8%, respectively. The overall mean age was 9.7 years. An almost equal number of children below 10 years, in comparison with those 10 years or more, had already been brought to medical attention for obesity. The proportions of children with metabolic syndrome were comparable between groups 1 and 2 (26.8% and 27.9%, respectively). There were no significant differences in terms the individual parameters of metabolic syndrome between groups 1 and 2 in terms of DBP (p=0.477), TC (p=0.082), HDL-C (p=0.475), LDL-C (p=0.163) and FPG (p=0.118). Group 2 subjects had significantly higher BMI, waist circumference, SBP and TG (Table 1).
Univariate analysis showed 3 clinical and 2 biochemical factors significantly associated with age group, with higher values in older obese patients ≥10 years old. These were SBP >130 mmHg (p=0.003), BP >130/85 (p=0.024), BMI >30 kg/m2 (p=0.007), TC ≥5.0 mmol/L (p=0.05) and TG >1.7 mmol/L (p=0.08) (Tables 2 and 3). Multivariate analysis (simple logistic regression) revealed that significant clinical and biochemical parameters of metabolic syndrome associated with age ≥10 years old were BMI >30 kg/m2 (OR 3.43, 95% CI, 1.37 to 8.54), systolic hypertension (OR 8.20, 95% CI, 1.74 to 38.7) and TC ≥5.0 mmol/L (OR 2.51, 95% CI, 0.97 to 6.47) (Table 4). However, analysis of the final model (multiple logistic regression) showed that only systolic hypertension (adjusted OR 7.17, 95% CI, 1.48 to 34.8) and BMI >30 kg/m2 (adjusted OR 3.02, 95% CI, 1.16 to 7.86) were significant (Table 5).
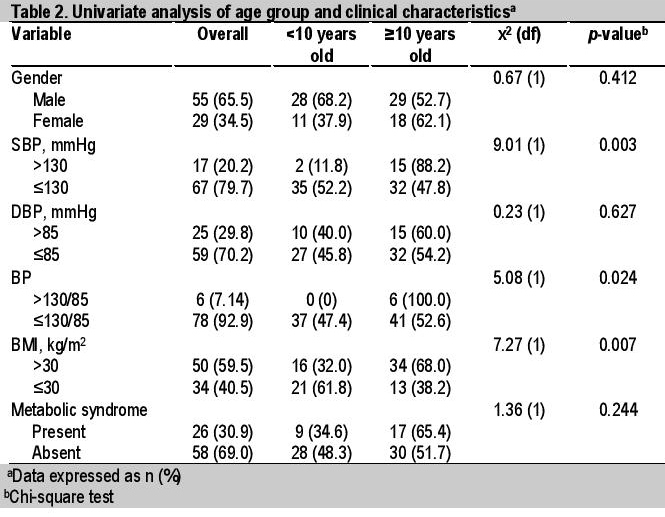
Table 2. Univariate analysis of age group and clinical characteristics
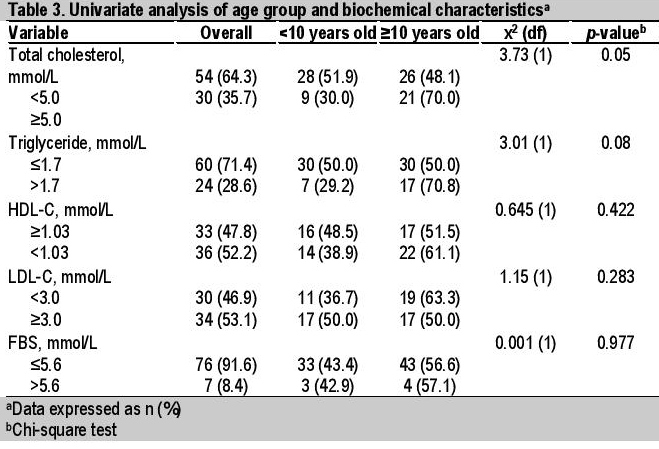
Table 3. Univariate analysis of age group and biochemical characteristics
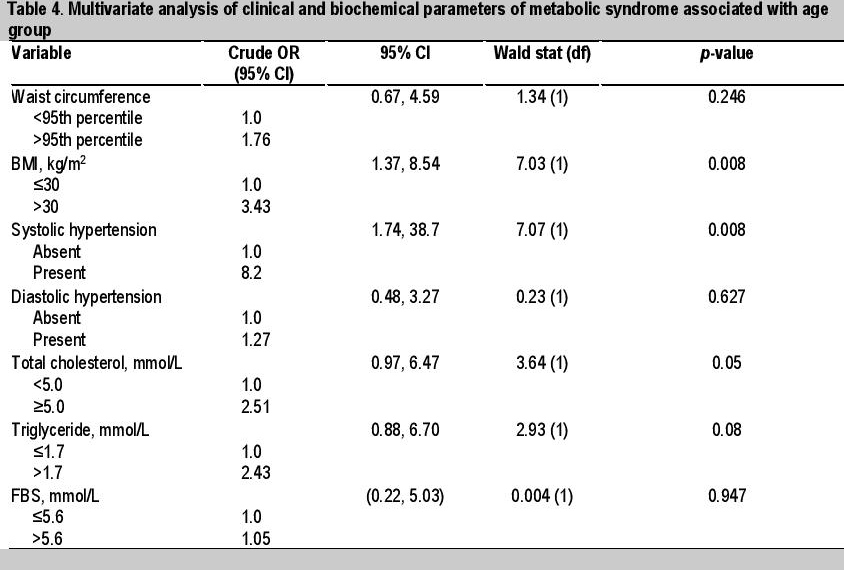
Table 4. Multivariate analysis of clinical and biochemical parameters of metabolic syndrome associated with age group
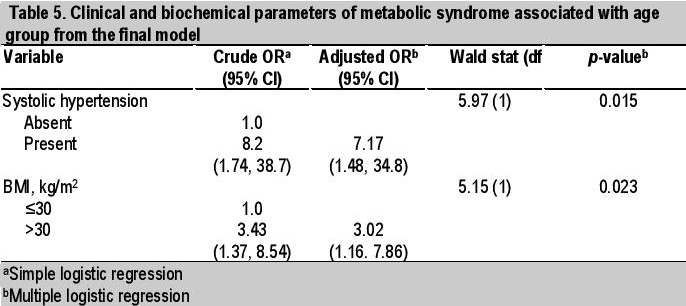
Table 5. Clinical and biochemical parameters of metabolic syndrome associated wuth age group from the final model
To determine the correlation of factors associated with metabolic syndrome and age groups, Spearman’s rank correlation was selected in view of the data’s non-normal distribution (coefficient of variation >10%). The test demonstrated a significant positive correlation between age group and BMI [rho (ρ)=0.379, p<0.01], SBP (𝞺=0.432, p<0.01) and waist circumference (𝞺=0.416, p=0.002). There was no significant correlation between age group and DBP (𝞺= 0.216, p=0.051), TG (𝞺=0.161, p=0.145), HDL (𝞺=-0.059, p=0.633) or FPG (𝞺=0.095, p=0.392).
The proportions of obese younger children (<10 years old) compared to older subjects (≥10 years old) were almost equal (48.9% and 51.1%, respectively). Comparison of characteristics showed similar clinical and biochemical parameters of metabolic syndrome in both age groups, with the exception of BMI, waist circumference, SBP and TG. These were further supported by positive correlation coefficients (𝞺) between age group and BMI, systolic hypertension and waist circumference. These findings may possibly be explained by the inclusion criteria specifying BMI consistent with morbid obesity (>30 kg/m2). The higher the BMI, the more likely for comorbidities and complications associated with obesity to occur, regardless of age. Both groups had similar proportions of parameters associated with metabolic syndrome, which would explain the observed similar proportions of metabolic syndrome (Table 1).
Both chi-square and regression analyses showed that older subjects ≥10 years old had higher BMI (>30 kg/m2), systolic hypertension and higher TC. However, only clinical parameters (BMI >30 kg/m2 and systolic hypertension) were found to be significant in the final model of logistic regression analysis. Older subjects were 7 times more likely to have systolic hypertension than younger subjects, and 3 times more likely to have BMI >30 kg/m2. These may be explained by the ensuing insulin resistance syndrome associated with physiologic elevations in sex hormones during puberty and visceral adiposity as observed in the older age group. Sex hormones work synergistically with growth hormone during puberty to contribute to pubertal growth spurt and to a physiologic phase of insulin resistance syndrome.[15] Visceral adiposity is a known risk for insulin resistance syndrome due to direct flux of fatty acids into the portal vein, which impairs insulin cascade signaling. Physiologic mechanisms associated with hypertension due to insulin resistance syndrome include sodium and water retention, systemic vasoconstriction and sympathetic stimulation.[16]
Similar studies by the groups of Atabek and Sangun in Turkey reported a significant difference in the proportion of obese prepubertal compared to pubertal children with metabolic syndrome (20% versus 37.6% and 33.1% versus 46.6%, p<0.001, respectively).[17],[18] In contrast, we found the proportions of obese children with metabolic syndrome in both age groups were not significantly different (𝞆2=0.012, p=0.091)(Table 2). On the other hand, our findings were also corroborated by the group of Rodrigues who found no statistically significant difference in the prevalence of metabolic syndrome between children (47.2 to 51.9%) and adolescents (48.1 to 52.8%).[19]
Metabolic syndrome was seen in 27.4% in the overall study population based on the IDF criteria. This is consistent with the findings of other studies that reported prevalence rates of metabolic syndrome ranging from 26.9 to 38.6% among obese children and adolescents, despite varied diagnostic criteria.[20],[21] Compared to other criteria which employed percentile values, the IDF diagnostic criteria for metabolic syndrome set higher cutoff values. A comparison of 3 diagnostic criteria for metabolic syndrome done by Sangun et al reported similar proportions of metabolic syndrome (31 to 39%) among obese children and adolescents.[18] Similarly, a systematic review by Friend et al., also observed a median prevalence rate of 29.2% of metabolic syndrome among obese children.22 On the other hand, a local study by the group of Quah, which utilized the IDF classification for metabolic syndrome, reported a prevalence rate of only 2.9% among their 34 obese patients.[10] Other local authors also reported relatively low proportions of metabolic syndrome (11.9% and 5.3% respectively) among their obese participants.[11],[12] On analysis, the number of obese children included by the group of Quah was relatively small, while the study by Fadzlina focused exclusively on 13-year old patients.[10],[12] Additionally, the observed differences in our prevalence rates of metabolic syndrome compared to those by the groups of Quah and Fadzlina could be due to different environmental characteristics: patients referred to our hospital-based study (USM) came from diverse backgrounds, including those from rural areas, in contrast to their studies which were conducted in an urban center (Kuala Lumpur).
There was no statistically significant difference in the proportion of obese children with metabolic syndrome by gender (𝞆2=0.28, p=0.596)(Table 2). However, there was a higher proportion of obese girls diagnosed with metabolic syndrome in comparison to obese boys (37.9 and 27.2%, respectively). A previous study by Ferreira et al also showed a significantly higher proportion of adolescent girls with metabolic syndrome (𝞆2=3.88, p= 0.049)(Table 2), which the authors attributed to the earlier age of onset of puberty and the associated physiologic reduction in insulin sensitivity in girls.[23],[24]
It is noteworthy that almost half of the obese children in our study were less than 10 years of age (n=41, 48.8%). The detection of risk factors of metabolic syndrome even in prepubertal (<10 years old) children is especially alarming, as it is anticipated that puberty, with its attendant increase in insulin resistance will increase the predisposition of these already at-risk children for the development of metabolic syndrome.[8] Early identification of these at-risk children by primary healthcare providers and prompt referral for early intervention may prevent the dire consequences of this burgeoning public health problem.[25]
The IDF criteria for the diagnosis of metabolic syndrome was utilized in our study as the diagnostic methods were readily available and fairly cost effective. While BMI is a relatively sensitive and specific measure of childhood obesity, it is less suited for the assessment of visceral adiposity.[26],[27] On the other hand, waist circumference is an acceptable measurement for the inference of visceral adiposity, as well as a surrogate for insulin resistance.[15] Obese children with similar BMI for age and sex may not necessarily have the same risk of developing obesity-related metabolic comorbidities or metabolic syndrome due to differences in body fat distribution and its consequent effects on the degree of insulin resistance.[28] While standardized reference charts for body mass index are readily available from the WHO and the CDC, there are no similar charts for waist circumference. Comparisons of different studies may be difficult, as each employ reference charts designed for a specific population. Waist circumference reference values for this study were derived from those prepared by Wee et al.[11]
The prevalence rate of metabolic syndrome among obese children in our population may be an underestimate due to the reliance of the IDF criteria on measurement of waist circumference, which is not assessed consistently during the review of records of children referred for obesity. We found that only 54 (64.3%) of our subjects had their waist circumference recorded. The primary drawback of the retrospective nature of this study is the incomplete data from the available patient records, as only 84 of the 101 obese children identified from hospital records were available. Furthermore, complete data was not available in all the 84 identified patient records. Patient history and measurement of various clinical parameters may also not be as uniform as possible, as these were performed by different individuals over a wide range of time. Potentially relevant information, such as breastfeeding practices in infancy, duration of exercise and screen time, was not regularly documented. As the majority of sampled patients are Malay, conclusions derived from this study may not be applicable to other ethnic groups. Ideally, pubertal staging of the subjects should also be included in this study to properly stratify subjects into pre-pubertal and pubertal age groups. It was also not possible to track any progressive increase in the incidence of metabolic syndrome over the years in our study.
The clinical and biochemical parameters of metabolic syndrome were similar between those <10 years old and ≥10 years old, with the exception of BMI, waist circumference, systolic hypertension and TG level. The clinical parameters that were found to be significantly associated with age ≥10 years were BMI >30 kg/m2 and systolic hypertension. The prevalence rate of metabolic syndrome among obese children attending Paediatric clinic in Hospital Universiti Sains Malaysia was 27.3%, which was found to be comparable to findings from other studies conducted in developed countries. The alarming presence of components of metabolic syndrome even in children less than 10 years of age should serve as an impetus for intensifying efforts aimed at prevention of childhood obesity in the community.
Statement of AuthorshipAll authors certified fulfillment of ICMJE authorship criteria.
Author DisclosureThe authors declared no conflict of interest.
Funding SourceNone.