The Multicenter, Open-Label, Observational LEAD-Ph Study: Real-World Safety and Effectiveness of Liraglutide in Filipino Participants with Type 2 Diabetes
Cecilia Jimeno,* Sjoberg Kho,** Grace Ko de los Santos,*** Neslie Buena-Bobis,+ Michael Villa++
Cecilia A. Jimeno, MD
Department of Pharmacology and Toxicology,
University of the Philippines College of Medicine,
Salcedo Hall, Pedro Gil Street, Ermita, Manila, Philippines 1100
Tel. No.: +632-426-4248
Fax No.:+632-556-1183
E-mail: cajimeno@up.edu.ph
ORCID: https://orcid.org/0000-0002-7658-0123
e-ISSN 2308-118x
Printed in the Philippines
Copyright © 2018 by the JAFES
Received July 23, 2018. Accepted September 12, 2018.
Published Online First: October 9, 2018.
Objective. Assess safety and effectiveness of liraglutide among Filipino participants with type 2 diabetes (T2D) in routine clinical practice.
Methodology. A 26-week, prospective, multicenter, open-label, observational study was conducted in adults with T2D prescribed liraglutide (1.2 mg or 1.8 mg) in routine clinical practice in the Philippines. Primary endpoint: incidence rate and type of serious adverse drug reactions (SADRs). Secondary endpoints included other aspects of safety, and effectiveness.
Results. Participants (n=1056) had a mean (standard deviation) age of 53.2 (12.0) years, and glycated hemoglobin (HbA1c) level of 8.8% (2.0). Of 19 ADRs reported in 17 participants, none were SADRs (primary endpoint). No serious adverse events were reported. From baseline to week 26: the proportion of participants with major hypoglycemic episodes (requiring assistance) decreased from 2.0% to 0.2%; and with minor episodes (plasma glucose <3.1 mmol/L [<56 mg/dL]) decreased from 6.1% to 1.5%; serum creatinine remained unchanged. Among secondary effectiveness endpoints, improvements were seen from baseline to week 26 in HbA1c level, fasting and postprandial blood glucose levels, body weight, blood pressure, and fasting lipid profile.
Conclusion. During routine clinical use of liraglutide for T2D in the Philippines, no new safety concerns were identified and blood glucose was lowered effectively.
Keywords: glucagon-like peptides, liraglutide, type 2 diabetes mellitus, safety, observational study, PhilippinesCore pathophysiologic defects in the progression of type 2 diabetes (T2D) include progressive pancreatic β-cell failure resulting in insulin deficiency and increased tissue insulin resistance compounding the problem.[1] It has been recognized that impairments in the incretin effect (in which insulin secretion is amplified by incretin hormones in response to the ingestion of glucose), particularly the action of glucagon-like peptide 1 (GLP-1), also play a key role in the progression of T2D[2],[3] and may be an important contributor to the observed hyperglycemia.[4] Targeting this impairment can form part of a dynamic management approach to counteract the progressive nature of T2D.[5],[6] GLP-1-mediated effects include glucose-dependent stimulation of endogenous insulin secretion, modulation of glucagon secretion, reduced appetite and food intake, delayed gastric motility and emptying, inhibition of β-cell apoptosis and improvement of β-cell function.[4],[7] Multiple physiologic effects of GLP-1, beyond glucose metabolism, mean that drugs that mimic the action of GLP-1 can target several core pathophysiological defects underlying the development of T2D.[7],[8] GLP-1 receptor agonists (GLP-1 RAs) are therefore among the second-line treatment options for T2D according to the treatment algorithm from the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD), and can be added after 3 months of initial monotherapy if the target glycated hemoglobin (HbA1c) level is not achieved.[9] Furthermore, the American Association of Clinical Endocrinologists (AACE) includes GLP-1 RAs as an acceptable first-line monotherapy – an alternative to metformin for patients with mild hyperglycemia (HbA1c <7.5%).[10]
The GLP-1 RA liraglutide has 97% homology to native GLP-1, with the addition of a fatty acid side chain that confers a substantially prolonged half-life, enabling once-daily dosing.[11] The efficacy and safety of liraglutide as monotherapy or in combination with oral antidiabetic drugs (OADs) were demonstrated in the randomized, controlled trials of the Liraglutide Effect and Action in Diabetes (LEAD) study series.[12],[13],[14],[15],[16],[17] Compared with active comparators in these trials, liraglutide generally improved HbA1c, fasting plasma glucose, postprandial blood glucose (PPBG) levels, and β-cell function. It also demonstrated favorable effects on body weight.[12]-[17] Improvements in glycemic control and weight loss were sustained for up to 1–2 years of follow-up.[18],[19],[20] Based on findings from clinical trials, liraglutide was generally well tolerated with an acceptable incidence of hypoglycemic episodes; the most common adverse events (AEs) reported were gastrointestinal, including nausea, vomiting and diarrhea.[12]-[17],[21] However, the gastrointestinal AEs tended to be transient and their incidence subsided over time.[22] Liraglutide was approved for the treatment of T2D in Europe in 2009 and subsequently in the USA in 2010.[23],[24]
Studies of liraglutide in Asian populations with T2D to date include a double-blind active-control trial in participants from China, South Korea, and India,[25] an open-label active comparator trial in Chinese participants,[26] and an observational study in India.[27] Liraglutide was approved in the Philippines in February 2011 for the treatment of adults with T2D, as monotherapy or in combination with OADs and/or basal insulin when these agents, together with diet and exercise, do not provide adequate glycemic control.[28] Despite the studies, this approval, and the inclusion of GLP-1 RAs in the ADA/EASD and AACE guidelines (commonly referred to by healthcare practitioners in the Philippines),[9],[10] local guidelines do not include GLP-1 RAs,[29] and experience of liraglutide use in the Philippines remains limited.
This prospective study was undertaken as a post-marketing surveillance commitment to the Philippine Food and Drug Administration (FDA). The data provide an assessment of the safety and effectiveness of liraglutide 1.2 mg and 1.8 mg among the Filipino population in a clinical practice setting.
METHODOLOGYStudy design
This was a 26-week, prospective, open-label, observational study in participants with T2D who were prescribed liraglutide in routine clinical practice. The study was conducted from September 1, 2011 to July 26, 2013 in 85 study centers in the Philippines by specialists and primary care physicians with experience in the management of T2D. The study is registered with ClinicalTrials.gov (NCT01345734).
Participants provided written informed consent prior to any study-related activity. This study was approved by the Ethics Review Board of the University of the Philippines-Philippine General Hospital and was conducted in accordance with the Declaration of Helsinki, and the Guidelines for Good Pharmacoepidemiology Practices.
Patient inclusion and exclusion criteria
Participants were included if they were adults (aged ≥18 years) with T2D, either newly diagnosed and not on anti-diabetic medication or already receiving other antidiabetic medications, who required treatment intensification with liraglutide according to the clinical judgment of their treating physician and were capable of giving informed consent. Participants were excluded if they had type 1 diabetes, were or had previously been treated with liraglutide, were participating in another clinical study, had hypersensitivity to liraglutide or to any of the excipients (disodium phosphate dihydrate, propylene glycol, phenol, water for injections), were pregnant, breast-feeding or had the intention of becoming pregnant within the following 6 months, or had a high probability of being lost to follow-up during the study period, as assessed by the treating physician.
Treatment
Liraglutide was administered (when deemed clinically warranted by the treating physician) in accordance with approved local labeling, as monotherapy or in combination with one or more OADs (dipeptidyl peptidase-4 inhibitors and/or exenatide therapy were discontinued at baseline, prior to liraglutide initiation) and/or basal insulin. Liraglutide was started at a dose of 0.6 mg once daily and increased by 0.6 mg, at intervals of at least 1 week, to a maintenance dose of 1.2 or 1.8 mg once daily by the investigator according to approved prescribing information. The study drug was self-administered at any (consistent) time, independent of meals, using an injection pen device.
Assessments
Visits occurred at baseline (Visit 1), at approximately 13 weeks (Visit 2) and finally at approximately 26 weeks (Visit 3). The suggested frequency and timing of visits were based on accepted clinical practice for the management of T2D. Further, any procedure carried out during this study was conducted according to routine practice.
Participants were instructed to maintain a diary to record AEs and hypoglycemic episodes. Investigators asked about adverse drug reactions (ADRs), serious adverse events (SAEs), and medical events of special interest (MESIs; which included major and minor hypoglycemic episodes) at study visits and additionally gathered information on laboratory assessments (fasting lipid profile values and serum creatinine levels). ADRs were considered to be AEs (any undesirable medical event) for which a causal relationship with the product was suspected (i.e., judged possible or probable by the reporting healthcare professional) and any abnormalities from laboratory assessments that were regarded as clinically significant (i.e., of a severity requiring active management). Serious ADRs (SADRs) and SAEs were considered to be AEs or ADRs that resulted in death, a life-threatening experience, hospitalization, persistent or significant disability/incapacity, a congenital anomaly/birth defect, or which required intervention to avoid one of these outcomes. As standard, MESIs were considered to be events such as medication errors or transmission of an infectious agent via the study drug; in this study, MESIs additionally included pancreatitis, thyroid gland disorders, neoplasms, and major hypoglycemia. Major hypoglycemia was defined as episode of hypoglycemia that the patient was unable to self-treat (including those events requiring administration of glucagon or intravenous glucose by another person); plasma glucose levels prior to the incident were recorded if available. (Minor hypoglycemia applied to events in which the patient was able to self-treat and for which plasma glucose <3.1 mmol/L [<56 mg/dL] was recorded. Episodes of minor hypoglycemia were reported in the case report form [CRF].)
Participants were also asked to record concomitant medications, and self-monitored blood glucose (SMBG) levels in their diaries. Most recent values of HbA1c, fasting blood glucose (FBG), and PPBG and the dates of measurement since the last visit prior to starting liraglutide treatment (if available) were obtained from patient medical records at baseline. Most recent values of HbA1c, FBG, and PPBG and date of measurement since last visit were obtained at study Visits 2 and 3. Serum creatinine levels, systolic blood pressure (SBP) and diastolic blood pressure (DBP) were recorded at each visit only when available from the patient’s medical record or patient recall.
Endpoints
The primary endpoint was the incidence of SADRs during the study period (26 weeks of liraglutide therapy). Secondary safety endpoints comprised: numbers of SAEs and ADRs during the study period; numbers of major hypoglycemic events registered during the 13 weeks preceding each study visit and minor hypoglycemic events registered during the 4 weeks preceding each study visit, in both cases during liraglutide therapy; changes in serum creatinine levels, SBP and DBP.
Secondary effectiveness endpoints comprised: changes in HbA1c (by visit, and change from baseline to week 13 and week 26); percentages of participants reaching HbA1c targets of ≤6.5% and <7.0% at the end of the study; changes in FBG and PPBG levels (after breakfast, lunch, or dinner); and changes in body weight and related parameters (body mass index [BMI], waist circumference, and hip circumference) (by visit, and changes from baseline to week 13 and week 26). Other parameters monitored were changes, if any, in fasting lipid profile (by visit), frequency of SMBG (at baseline and week 26), and the prescription of antidiabetic (at baseline before and after initiation of liraglutide), antihypertensive, and lipid-lowering medications (by visit).
Statistical analysis
A sample of 812 participants was required to obtain a 95% confidence interval (CI) (±1.5%) for an estimated SADR incidence of 5%. To allow for an expected 20% discontinuation rate, recruitment of 1000 participants was planned. Statistical analyses were performed using SAS version 9.3 (SAS Institute, Cary, NC, USA). Differences across visits for continuous variables (such as weight, HbA1c, FBG, or PPBG) were analyzed using a one-way analysis of variance (ANOVA) with study visit as a classification variable (two-sided p value). For continuous variables that reached statistical significance across visits using ANOVA, differences between study visits were then analyzed using a one-sided Dunnett’s test and the p value presented (lower tail [except high-density lipoprotein cholesterol, upper tail]). Changes from baseline were expressed as least squares means with standard error (SE) and 95% CI from one-way ANOVA and, where appropriate, a one-sided p-value from the Dunnett’s test. The number of hypoglycemic events was analyzed using the Wilcoxon signed-rank test. Discrete variables were summarized using frequency tables (N, %). Proportions of participants with missing data were indicated as % where appropriate.
Participants
Of 1056 participants enrolled and exposed to liraglutide (full analysis set [FAS]), 75.2% completed the study and were included in the effectiveness analysis set (EAS) (Figure 1). Commonly cited reasons for non-completion were discontinuation of liraglutide (n=137), lost to follow-up (n=115), and ADRs (n=5).
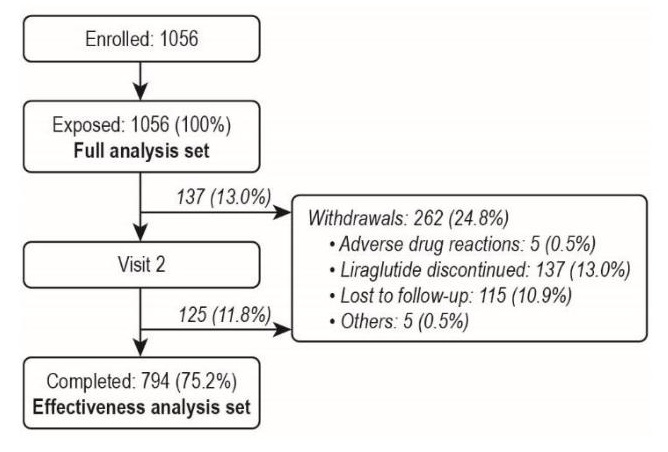
Figure 1. Participant disposition.
Baseline demographic and disease characteristics are shown in Table 1. There was a higher proportion of females than males (52.9% versus 47.1%). The population had a mean (SD) age of 53.2 (12.0) years, HbA1c of 8.8% (2.0), and diabetes duration of 9.2 (8.0) years. The most frequent diabetes complications at baseline were peripheral neuropathy (19.7%) and coronary heart disease (16.6%). The most frequently cited reason given by participants as to why they accepted the physician’s suggestion for starting liraglutide was to improve weight control (cited by 90.2% of participants) (Table 2).
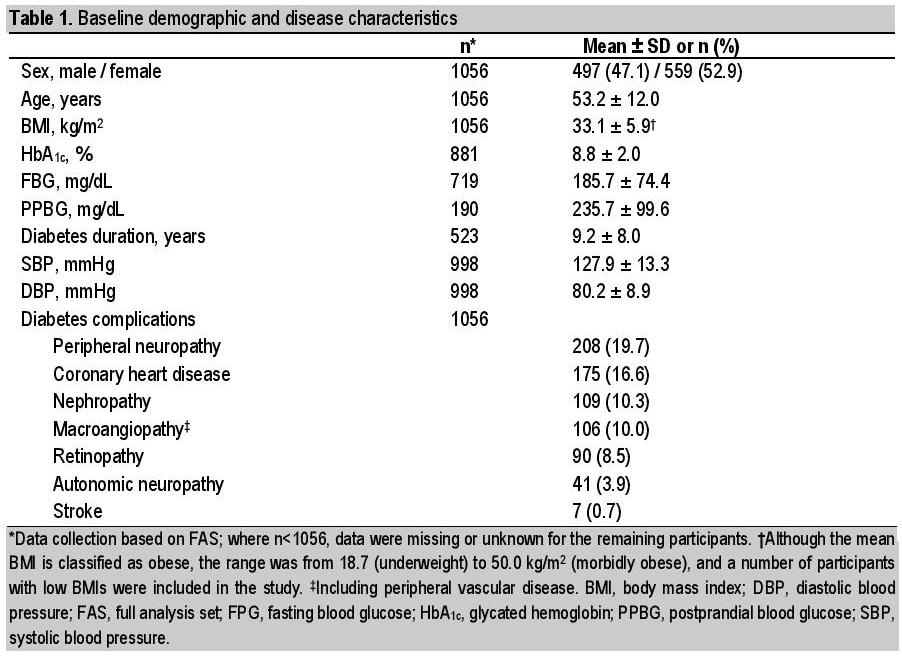
Table 1. Baseline demographic and disease scharacteristic
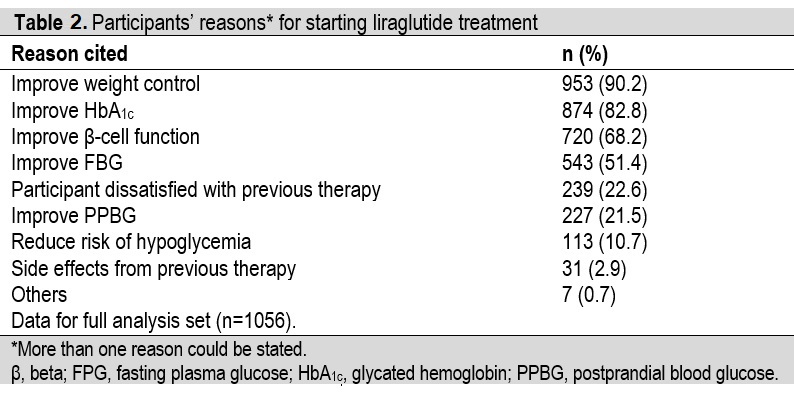
Table 2. Participants' reasons* for starting liraglutide treatment
Safety
SADRs, SAEs and ADRs
No SADRs were reported during the study (primary endpoint), and there were also no reports of SAEs. There were 19 reported ADRs from 17 participants (Table 3). Although hypoglycemia events were to be reported on the CRF, separately from ADRs, four events of hypoglycemia were in fact recorded as ADRs; as ADRs, severities were thus not classified as major or minor hypoglycemia but instead as mild, moderate, or severe (three events were considered mild, one was moderate). Contrary to the protocol, an AE that was neither considered related to study drug (i.e., an ADR) nor serious (i.e., an SAE) was also recorded. This AE, frozen shoulder (periarthritis), occurred in a 33-year-old patient, was considered to be moderate in severity, and the patient did not recover fully by the end of the study.
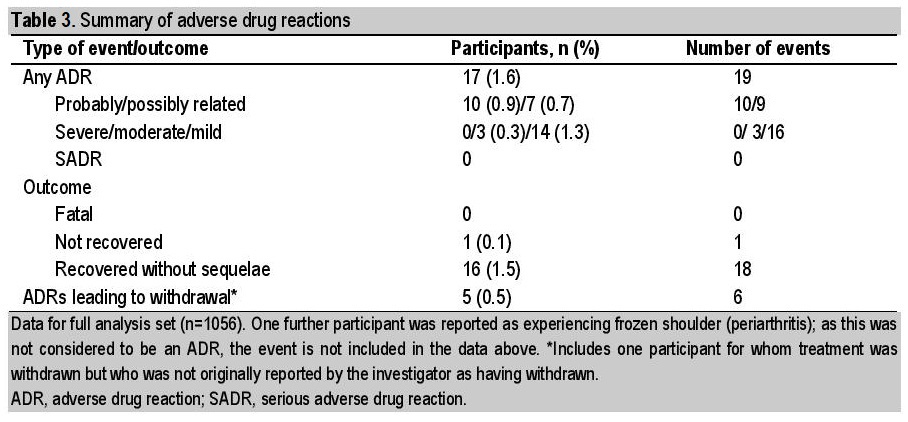
Table 3. Summary of adverse drug reactions
The majority (16/19) of ADRs were mild, and all but one of the participants recovered fully. The exception was a 51-year-old participant with a mild case of diarrhea, deemed possibly related to study drug and for whom liraglutide was permanently withdrawn. A further four participants also withdrew from the study due to ADRs, specifically cushingoid features (moon face), hypoglycemia, nausea and vomiting, and dizziness. The participant with dizziness was not originally reported as a treatment withdrawal by the investigator but, because liraglutide was permanently discontinued, the participant has been included in these analyses.
Other than the four events of hypoglycemia inadvertently included as ADRs, the most commonly reported ADRs by system organ class (Table 4) were gastrointestinal disorders (five events in three participants [0.3%]: two ADRs of vomiting and one each of diarrhea, nausea, and upper abdominal pain) and general disorders and administration-site conditions (three events in three participants [0.3%]: fatigue, injection-site rash, and malaise).
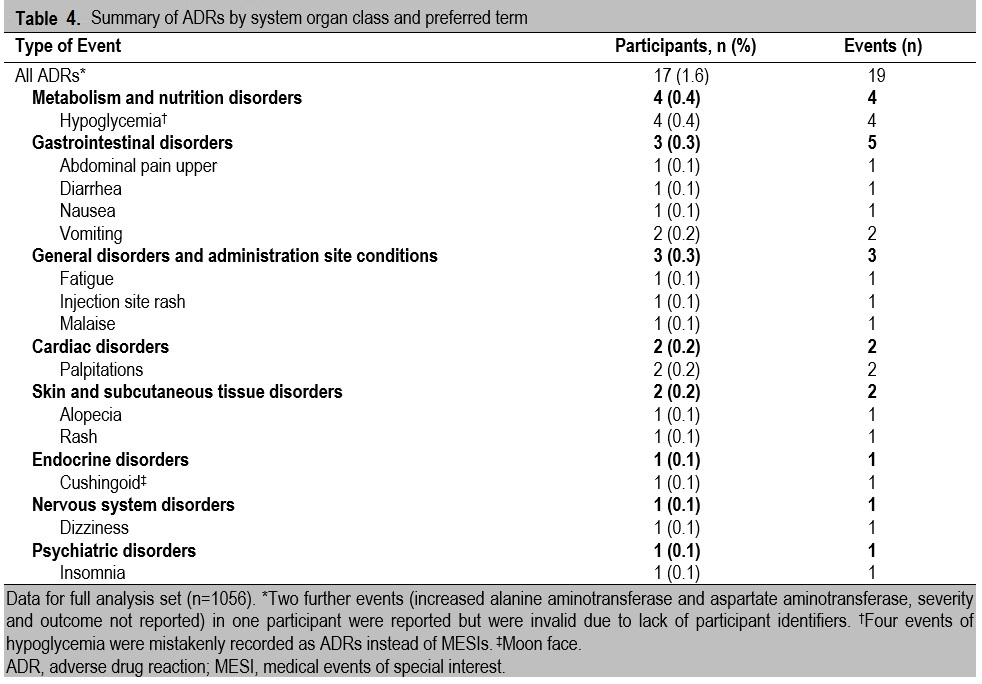
Table 4. Summary of ADRs by system organ class and preferred term
Hypoglycemia
The proportion of participants reporting hypoglycemic episodes decreased during the study period. At baseline (i.e., for the 13 weeks preceding initiation of treatment with liraglutide), the proportion of participants with major hypoglycemic episodes was 2.0% (21/1056); this decreased to 0.6% (6/1056) of participants at week 13, and decreased further to 0.2% (2/1056) at week 26. The proportion of participants with minor hypoglycemic episodes at baseline was 6.1% (64/1056); this decreased to 2.5% (26/1056) of participants at week 13, and to 1.5% (16/1056) at week 26. It is important to note, however, that at each of these timepoints data were missing or unknown for a varied proportion of participants (ranging from 5.4 to 24.8%). Furthermore, these events did not include the four episodes reported as ADRs.
Serum creatinine
Differences in serum creatinine during the course of the study did not reach statistical significance (mean [SE] change from baseline to week 26 was 0.02 ± 0.02 mg/dL [95% CI: –0.03; 0.06]; p=0.2327 [ANOVA], FAS, n=183).
Effectiveness
Glycemic controlThere was a significant reduction in HbA1c levels from baseline to week 26 with liraglutide treatment (Figure 2); the mean (SE) change from baseline in HbA1c was 1.81% ± 0.05 [95% CI: 1.92; 1.71], p<0.0001 (n=577; baseline to week 26). The target HbA1c level <7.0% (the goal identified by the ADA/EASD) was reached by 197/794 (24.8%) participants in the EAS at week 13 (data missing for 171/794 [21.5%] participants) and by 322/794 (40.6%) participants at week 26 (missing data: 151/794 [19.0%] participants). The proportions of participants achieving HbA1c levels ≤6.5% (the AACE goal) at weeks 13 and 26 were 126/794 (15.9%) and 234/794 (29.5%), respectively (missing data as for target <7.0%).
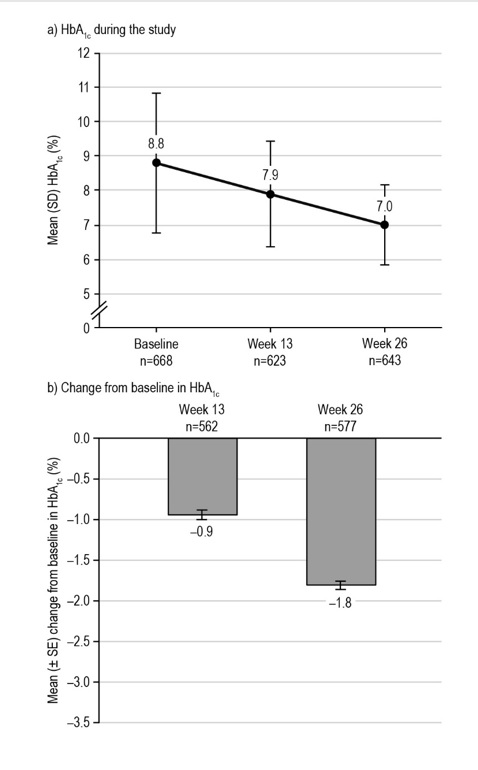
Figure 2. Glycemic control during the LEAD-Ph study: HbA1c. From participants in the EAS with available data. The mean (± SE) change from baseline in HbA1c level at week 13 was –0.94 ± 0.06%, p<0.0001* (n=562), and –1.81 ± 0.05%, p<0.0001* (n=577) at week 26. *Statistical difference at the 5% level, one-tailed. EAS, effectiveness analysis set; HbA1c, glycated hemoglobin; SD, standard deviation; SE, standard error.
FBG levels were also significantly reduced from baseline to week 26 with liraglutide treatment (Figure 3), with a mean (SE) change of 67.61 ± 3.26 mg/dL [95% CI: 74.00; 61.22], p<0.0001 (n=412). Similarly, for the few participants with PPBG assessments after the same meal (breakfast) at each study visit available for analysis, there was a significant reduction in PPBG levels across visits with liraglutide treatment (Figure 4). The mean (SE) change in post-breakfast PPBG (baseline to week 26) was 109.27 ± 14.01 mg/dL [95% CI: 137.02; 81.52], p<0.0001 (n=51). Nevertheless, these PPBG data should be interpreted with caution, due to the low numbers of participants with data available (n=51–67).
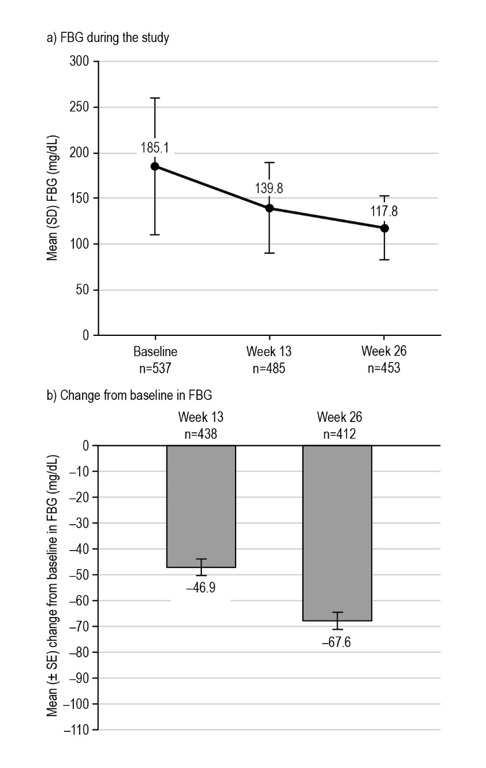
Figure 3. Glycemic control during the LEAD-Ph study: FBG. From participants in the EAS with available data. The mean (± SE) change from baseline in FBG level at week 13 was −46.94 ± 3.16 mg/dL, p<0.0001* (n= 438), and −67.61 ± 3.26 mg/dL, p<0.0001* at week 26 (n=412). *Statistical difference at the 5% level, one-tailed. EAS, effectiveness analysis set; FBG, fasting blood glucose; SD, standard deviation; SE, standard error.
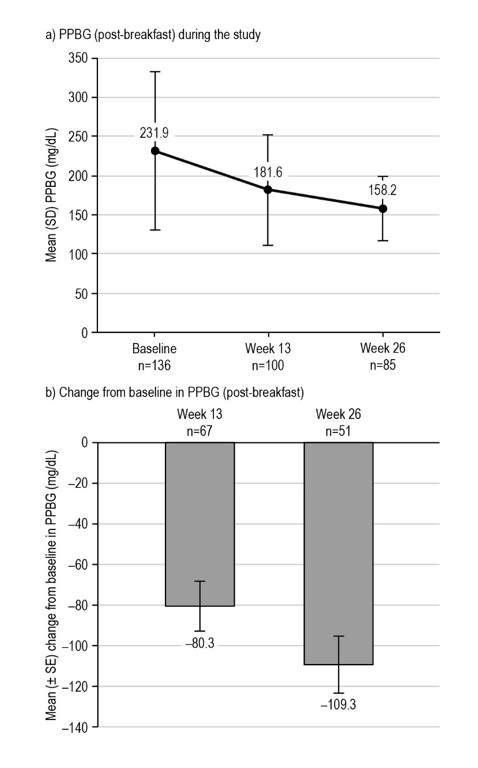
Figure 4. Glycemic control during the LEAD-Ph study: PPBG. From participants in the EAS with available data. The mean (± SE) change in post-breakfast PPBG level at week 13 was −80.32 ± 12.23 mg/dL, p<0.0001* (n=67), and −109.27 ± 14.01 mg/dL, p<0.0001* at week 26 (n=51). *Statistical difference at the 5% level, one-tailed. EAS, effectiveness analysis set; PPBG, postprandial blood glucose; SD, standard deviation; SE, standard error.
Body weight, BMI and body measurements
Body weight decreased during the study (Figure 5, Table 5). Accordingly, there was a concomitant significant change in BMI from baseline to week 26, and mean (SE) change of 1.37 ± 0.04 kg/m2 [95% CI: 1.44; 1.29], p<0.0001 (both EAS, n=794). Participants with a higher BMI at baseline lost more weight during the study than participants with a lower baseline BMI. Participants with BMI ≥35 kg/m2 had a significant reduction in body weight (baseline to week 26) with a mean (SE) weight change of 4.35 ± 0.19 kg [95% CI: 4.73; 3.98], p<0.0001 (n=291), compared with participants with BMI <25 kg/m2 having a mean (SE) weight change of 2.05 ± 0.31 kg [95% CI: 2.65; 1.44] (n=53), which did not reach statistical significance. Waist and hip circumference measurements tended to decrease in participants with available data, although the participant numbers were low for these endpoints and differences did not reach statistical significance (Table 3).
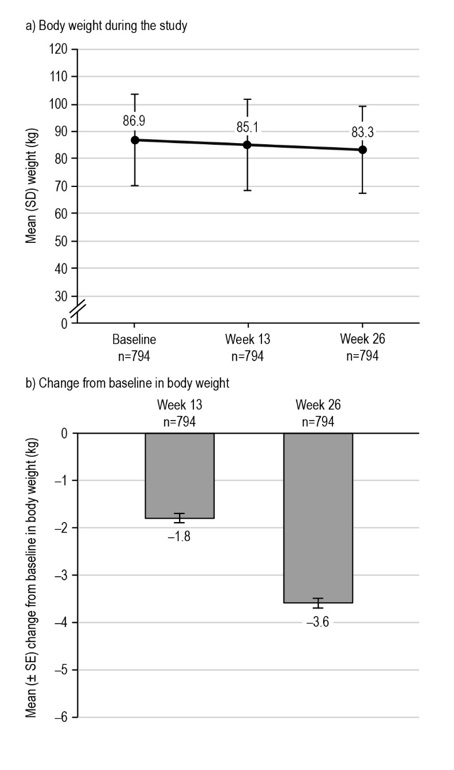
Figure 5. Changes in body weight during the LEAD-Ph study. From participants in the EAS with available data. The mean (± SE) change from baseline in body weight at week 13 was −1.79 ± 0.10 kg, p=0.0267* (n=794), and −3.58 ± 0.10 kg, p<0.0001* at week 26 (n=794). *Statistical difference at the 5% level, one-tailed. EAS, effectiveness analysis set; SD, standard deviation; SE, standard error.
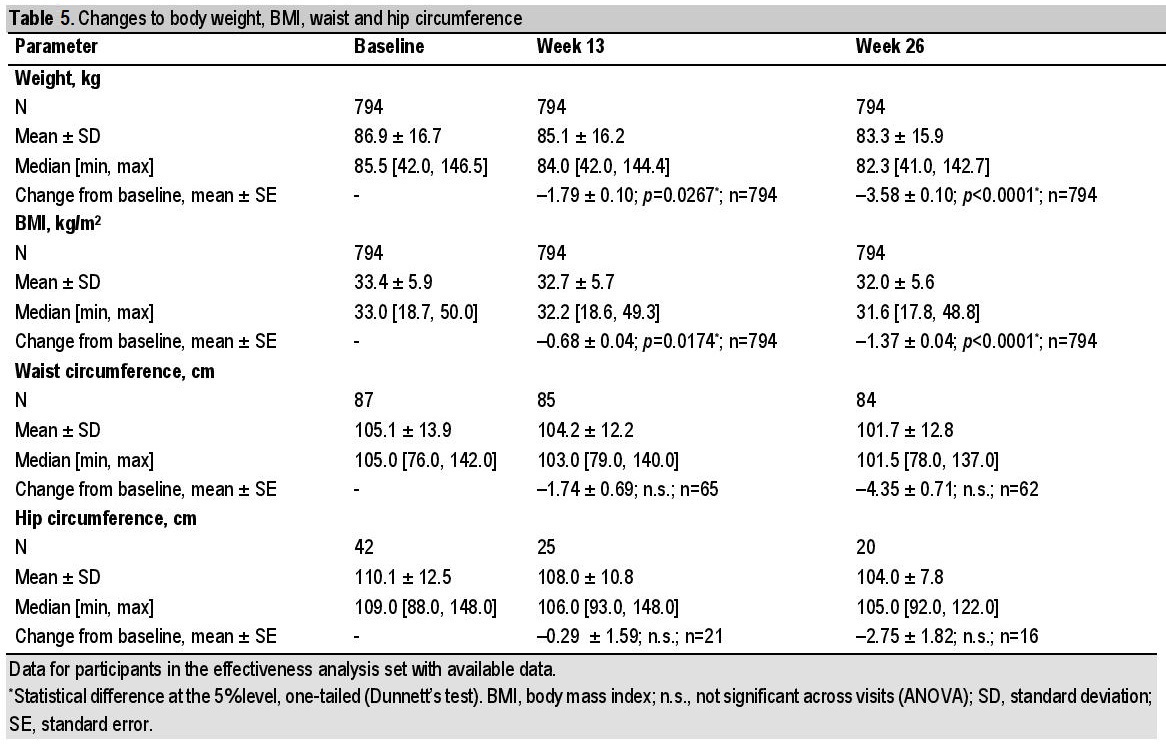
Table 5. Changes to body weight, BMI, waist and hip circumference
Blood pressure
Mean (SE) changes for SBP were 3.11 ± 0.41 mmHg [95% CI: 3.91; 2.30], p<0.0001 (n=750; baseline to week 13), and 5.31 ± 0.41 mmHg [95% CI: 6.12; 4.50], p<0.0001 (n=746; baseline to week 26). Mean (SE) changes for DBP were 2.93 ± 0.32 mmHg [95% CI: 3.57; 2.30]; p<0.0001 (n=750; baseline to week 13), and 5.35 ± 0.32 mmHg [95% CI: 5.99; 4.71]; p<0.0001 (n=746; baseline to week 26).
Fasting lipid profile
Improvements in fasting lipid profiles were shown across the variables studied (Table 6). In the EAS, the mean (±SE) change in total cholesterol was 45.56 ± 3.55 mg/dL [95% CI: 52.53; 38.59]; p<0.0001 (n=179; baseline to week 26). High-density lipoprotein cholesterol increased by a mean (SE) of 4.50 ± 1.21 mg/dL [95% CI: 2.12; 6.88]; p<0.0001 (n=156; baseline to week 26), while the mean ± SE change in low-density lipoprotein cholesterol was 32.63 ± 3.56 [95% CI: 39.63; 25.63] mg/dL; p<0.0001 (n=158; baseline to week 26). Triglycerides similarly showed a reduction (mean [SE] change: 36.59 ± 5.40 mg/dL [95% CI: 47.21; 25.98]; p<0.0001 [n=162; baseline to week 26]).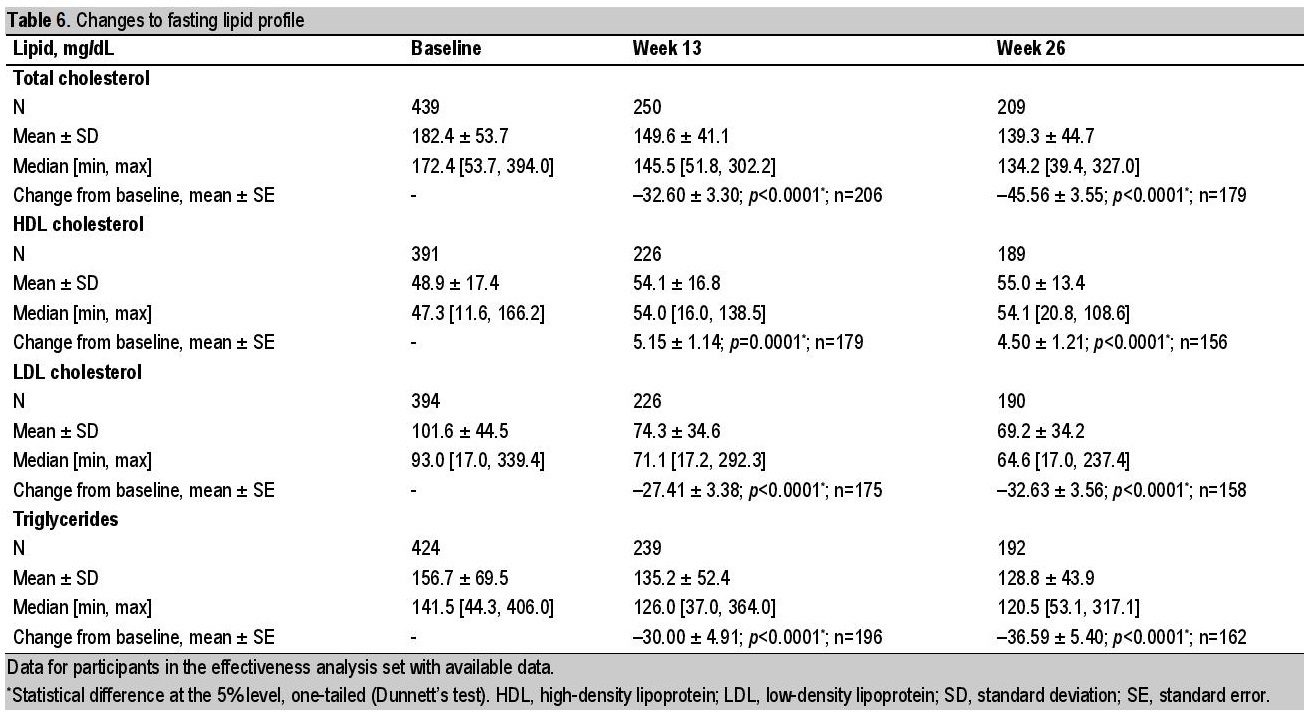
Table 6. Changes to fasting lipid profile
Diabetes management
Participants were asked to self-monitor their blood glucose levels; however, most did not. At baseline, a total of 301/794 (37.9%) participants reported checking their blood glucose levels. This number comprised 153 (19.3%) who checked daily, 118 (14.9%) who checked weekly, and 30 (3.8%) who checked monthly. The remaining 493/794 (62.1%) did not regularly monitor their blood glucose levels. At week 26, the proportions of participants who reported undertaking regular SMBG decreased slightly to a total of 265/794 (33.4%), with 136 (17.1%) reporting daily monitoring, 110 (13.9%) weekly and 19 (2.4%) monthly (with 66.6% not reporting regular monitoring).
Before beginning treatment with liraglutide, most participants were treated with a combination of OADs (including metformin) (438 [41.5%]) or OADs and insulin (277 [26.2%]) (Table 7). After initiation of liraglutide (at baseline), similar proportions of participants were treated with a combination of liraglutide, OADs and insulin (293 [27.7%]), liraglutide and metformin alone (290 [27.5%]), and liraglutide combined with more than one OAD including metformin (249 [23.6%]), while 58 (5.5%) participants were treated with liraglutide and insulin. A total of 166 (15.7%) participants were treated with liraglutide monotherapy.

Table 7. Antidiabetic therapy before and after liraglutide initiation (at baseline)
Changes in lipid-lowering and antihypertensive medications
Use of lipid-lowering medication remained fairly stable during the study. At baseline, 20.0% (148/739) of participants in the EAS were treated with fibrates, and 90.0% (665/739) were treated with statins. After 26 weeks of treatment with liraglutide, these proportions were 20.9% (89/426) and 89.4% (381/426), respectively.
At baseline, most participants (570/664, 85.8%) were being treated with angiotensin II receptor blockers. Over the course of the study, this proportion increased slightly to 93.9%, although from considerably fewer participants with available data (355/378). There were decreases in all other types of antihypertensive treatment (angiotensin-converting enzyme inhibitors, beta-blockers, calcium channel blockers, and diuretics; data not shown).
The LEAD-Ph study was a post-marketing surveillance commitment to the Philippine FDA, and was conducted to assess the safety and effectiveness of liraglutide over 26 weeks in participants with T2D during routine clinical practice in the Philippines. Overall, this study found no new safety concerns and demonstrated the clinical effectiveness of liraglutide in participants with T2D within this setting. There were no reports of SADRs (the primary endpoint), and participants treated with liraglutide for 26 weeks, as prescribed in the course of routine care, experienced statistically significant and clinically relevant improvements in HbA1c, FBG, and PPBG levels. No SAEs, and few ADRs, were reported (19 events in 17 participants), and the reported incidence of hypoglycemia was also low.
A higher frequency of ADRs and SADRs might have been expected in LEAD-Ph, based on other observational studies of liraglutide use in European participants with T2D.[30],[31] In a 12-month Belgian study, 58/245 (24%) of the participants reported ADRs and 6/245 (2.4%) reported an SADR, while in a French study, 458/3152 (14.5%) of the participants experienced an AE possibly related to liraglutide during 2 years of follow up.[30],[31] There are limited data on the frequency of treatment-related AEs in observational studies involving Asian populations. In a 26-week, open-label, active-comparator trial of liraglutide, of 183 Chinese participants treated with liraglutide, the proportion of patients with AEs possibly/probably related to treatment was 43.2%.[26] However, in the observational 26-week LEAD-In study of liraglutide in routine clinical use in India, only 19/1416 (1.3%) of the participants reported 20 AEs, of which 19 were considered to be treatment-related, and no participant reported an SADR, which resonates with the findings of LEAD-Ph.[27] Other than the four events of hypoglycemia, the most frequently reported ADRs by system class during LEAD-Ph were gastrointestinal disorders. This accords with the findings from the LEAD clinical trials for liraglutide and the safety profile of the GLP-1 RA class, in which gastrointestinal AEs are the commonly observed AEs.[12]-[17],[19],[21],[32],[33],[34] However, withdrawals due to gastrointestinal events are uncommon, as these events are generally transient and can be mitigated by weekly dose escalation.[12]-[17],[19],[21],[32],[33] During this study, 24.8% of participants in the FAS had withdrawn by week 26, approximately half of these withdrawals occurred by week 13, yet only 0.5% of participants in the FAS withdrew due to ADRs. Most withdrawals in LEAD-Ph were attributed to liraglutide discontinuations (13.0%), a reason for which may be financial constraints (participants were required to pay for liraglutide prescriptions and were not reimbursed in this observational study).
Notwithstanding similarities between LEAD-Ph and the Indian LEAD-In study, the absence of SADRs and the low frequency of ADRs for a study of the size of LEAD-Ph (n=1056 [FAS]) should be interpreted with caution. While training on safety reporting was provided to investigators at the start of the study and at site initiation visits, and repeated reminders were provided throughout the study, insufficient event reporting may have occurred. Additionally, the varied and significant proportion of participants with missing data may also have been a contributing factor.
The inadvertent reporting of an AE (frozen shoulder [periarthritis]) among the ADRs, and of four episodes of hypoglycemia as ADRs, suggests that there may have been confusion for some investigators regarding the reporting procedure. In addition, the study physicians may also have implemented slower titration protocols than was recommended in the package insert, with dose increases routinely separated by more than 1 week (data on liraglutide dose escalation were not collected during the study). Therefore, collection of data on liraglutide starting dose and dose changes, as well as compliance to therapy, might have provided additional insight for clinicians, and the absence of these data limits the conclusions that can be drawn from this study.
It is possible that a lower frequency of ADR reporting may occur generally in the Philippines and other Asian populations than in European countries where liraglutide has been studied, with perhaps less experience of the concept of pharmacovigilance monitoring. This supposition cannot be explored at this time, as there are no public databases from which to gather local data on ADR reporting, and studies examining the issue of underreporting are lacking. Thus, there are no solid data for comparison with other populations, but this gap in itself suggests that the importance of safety reporting might be less prominent in the Philippines than elsewhere.
The significant improvements in HbA1c, FBG, and PPBG levels while on treatment with liraglutide in this study provide confirmation of the real-world effectiveness of this treatment in Filipino participants with T2D. The change from baseline to week 26 in HbA1c levels (mean change 1.81%, p<0.0001, from 8.8% at baseline) was similar to that seen in LEAD-In (-1.6%, p<0.0001, from 8.8% at baseline),[27] and these effectiveness results are consistent with the LEAD clinical trials.[12]-[17],[19],[21],[31],[32] Given that liraglutide is also associated with a low risk of hypoglycemia,[35] it is perhaps not surprising that the incidence of hypoglycemia in the present study was low despite improvements in glycemic control. Importantly, the incidence was low even though 351/1056 (33.2%) participants were receiving concomitant insulin (with or without OAD); it is unclear to what extent Filipino participants receiving insulin may have additionally mitigated the risk of hypoglycemia by a habit of snacking. The trend towards weight reduction observed while on liraglutide among Filipino participants was also encouraging and is similar to that seen in the phase 3 LEAD clinical trials, with concomitant improvements in BMI and body measurements. Collectively, these findings are important for meeting physicians’ and patients’ expectations; improved weight control was the most frequently cited reason for starting liraglutide (90% of participants). Besides improvements in body weight and BMI, other clinical parameters included in this surveillance also point towards a lowering in the overall cardiovascular risk profile. Specifically, there were numerical improvements in fasting lipid profile, and there was a trend towards reductions in SBP and DBP.
In terms of the management of diabetes and use of concomitant medications, there were no notable changes during the study. A similar proportion of participants took OADs in combination with insulin before and after liraglutide initiation at baseline. The frequency of SMBG was low and reduced slightly from baseline to study end. Use of lipid-lowering and antihypertensive medication also remained fairly stable during the course of the study.
In addition to those limitations already mentioned, the varied and significant proportion of participants with missing data and high discontinuation rate (262/1056 [24.8%]) should be acknowledged. Further limitations include the absence of data for heart rate, which may be useful to include as an endpoint in subsequent studies, and self-monitoring of blood glucose was not performed by the majority of participants. Finally, this study was limited by the absence of a control group and lack of adjustment for missing data. It is also subject to the inherent limitations of its observational nature. Nevertheless, this study reports the first data on the use of liraglutide in the Philippines, and provides a useful insight into the safety and effectiveness of liraglutide in routine clinical practice among Filipino participants.
During this observational study of the routine clinical use of liraglutide for T2D in the Philippines, no SADRs were reported, less than 2% of participants reported an ADR, and the incidence of hypoglycemia was reduced at the end of the study compared with baseline. Improvements in glycemic control, body weight, BMI, blood pressure, and fasting lipid profile were also observed. There were no notable changes in the management of diabetes during the study, or notable changes in concomitant medication use after liraglutide initiation at baseline. In summary, no new safety concerns were identified with the routine clinical use of liraglutide as a treatment for T2D in the Philippines and effectiveness results were generally consistent with previous studies.
AcknowledgmentsThe authors thank Dr. Diana Edralin, Gurudutt Nayak, Dr. Mohd Nawi Wahid, Rashmi Jain and Sohan Dey (Novo Nordisk) and also Dr. Richard Elwyn Fernando and Balasubramanian L for their review and input into the manuscript.
Statement of AuthorshipAll authors certified fulfillment of ICMJE authorship criteria.
Author DisclosureDr. Cecilia Jimeno has attended advisory boards and speakers’ bureau for, and has received travel grants for CME from Novo Nordisk and is a member of a regional advisory board for liraglutide; she is also Vice-Editor of the Journal of the ASEAN Federation of Endocrine Societies. Dr. Sjoberg Kho has received honoraria for lectures and as an advisory board member with Novo Nordisk. Dr. Grace Ko de los Santos has no conflicts to report. Dr. Neslie Buena-Bobis was an employee of Novo Nordisk at the time of working on this article. Dr. Michael Villa declared no conflict of interest.
Funding SourceThe sponsor (Novo Nordisk) was involved in the study design and protocol development, reviewed the manuscript for scientific accuracy and provided statistical support. The development and submission of this manuscript was assisted by Tom Vizard of Watermeadow Medical, an Ashfield Company, part of UDG Healthcare plc, funded by Novo Nordisk.
[1] Kendall DM, Cuddihy RM, Bergenstal RM. Clinical application of incretin-based therapy: Therapeutic potential, patient selection and clinical use. Am J Med. 2009;122(6 Suppl):S37-50. PubMed CrossRef
[2] Holst JJ, Knop FK, Vilsbøll T, Krarup T, Madsbad S. Loss of incretin effect is a specific, important, and early characteristic of type 2 diabetes. Diabetes Care. 2011;34 Suppl 2:S251-7. PubMed PubMed Central CrossRef
[3] Holst JJ, Vilsbøll T, Deacon CF. The incretin system and its role in type 2 diabetes mellitus. Mol Cell Endocrinol. 2009;297(1-2):127-36. PPubMed CrossRef
[4] Holst JJ. The physiology of glucagon-like peptide 1. Physiol Rev. 2007;87(4):1409-39. PubMed CrossRef
[5] Bailey T. Options for combination therapy in type 2 diabetes: Comparison of the ADA/EASD position statement and AACE/ACE algorithm. Am J Med. 2013;126(9 Suppl 1):S10-20. PubMed CrossRef
[6] Nauck M. Incretin therapies: Highlighting common features and differences in the modes of action of glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors. Diabetes Obes Metab. 2016;18(3):203-16. PubMed PubMed Central CrossRef
[7] Meier JJ. GLP-1 receptor agonists for individualized treatment of type 2 diabetes mellitus. Nat Rev Endocrinol. 2012;8(12):728-42. PubMed CrossRef
[8] DeFronzo RA, Triplitt CL, Abdul-Ghani M, Cersosimo E. Novel agents for the treatment of type 2 diabetes. Diabetes Spectr. 2014;27(2):100-12. PubMed PubMed Central CrossRef
[9] Inzucchi SE, Bergenstal RM, Buse JB, et al. Management of hyperglycemia in type 2 diabetes, 2015: A patient-centered approach: Update to a position statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 2015;38(1):140-9. CrossRef
[10] Garber AJ, Abrahamson MJ, Barzilay JI, et al. AACE comprehensive diabetes management algorithm 2013. Endocr Pract. 2013;19(2):327-36. PubMed
[11] Knudsen LB, Nielsen PF, Huusfeldt PO, et al. Potent derivatives of glucagon-like peptide-1 with pharmacokinetic properties suitable for once daily administration. J Med Chem. 2000;43(9):1664-9. PubMed
[12] Marre M, Shaw J, Brändle M, et al. Liraglutide, a once-daily human GLP-1 analogue, added to a sulphonylurea over 26 weeks produces greater improvements in glycaemic and weight control compared with adding rosiglitazone or placebo in subjects with Type 2 diabetes (LEAD-1 SU). Diabet Med. 2009;26(3):268-78. PubMed PubMed Central CrossRef
[13] Nauck M, Frid A, Hermansen K, et al. Efficacy and safety comparison of liraglutide, glimepiride, and placebo, all in combination with metformin, in type 2 diabetes: The LEAD (liraglutide effect and action in diabetes)-2 study. Diabetes Care. 2009;32(1):84-90. PubMed PubMed Central CrossRef
[14] Garber A, Henry R, Ratner R, et al. Liraglutide versus glimepiride monotherapy for type 2 diabetes (LEAD-3 Mono): A randomised, 52-week, phase III, double-blind, parallel-treatment trial. Lancet. 2009;373(9662):473-81. PubMed CrossRef
[15] Russell-Jones D, Vaag A, Schmitz O, et al. Liraglutide vs insulin glargine and placebo in combination with metformin and sulfonylurea therapy in type 2 diabetes mellitus (LEAD-5 met+SU): A randomised controlled trial. Diabetologia. 2009;52(10):2046-55. PubMed PubMed Central CrossRef
[16] Buse JB, Rosenstock J, Sesti G, et al. Liraglutide once a day versus exenatide twice a day for type 2 diabetes: A 26-week randomised, parallel-group, multinational, open-label trial (LEAD-6). Lancet. 2009;374(9683):39-47. PubMed CrossRef
[17] Zinman B, Gerich J, Buse JB, et al. Efficacy and safety of the human glucagon-like peptide-1 analog liraglutide in combination with metformin and thiazolidinedione in patients with type 2 diabetes (LEAD-4 Met+TZD). Diabetes Care. 2009;32(7):1224-30. PubMed PubMed Central CrossRef
[18] Nauck M, Frid A, Hermansen K, et al. Long-term efficacy and safety comparison of liraglutide, glimepiride and placebo, all in combination with metformin in type 2 diabetes: 2-year results from the LEAD-2 study. Diabetes Obes Metab. 2013;15(3):204-12. PubMed CrossRef
[19] Pratley R, Nauck M, Bailey T, et al. One year of liraglutide treatment offers sustained and more effective glycaemic control and weight reduction compared with sitagliptin, both in combination with metformin, in patients with type 2 diabetes: A randomised, parallel-group, open-label trial. Int J Clin Pract. 2011;65(4):397-407. PubMed PubMed Central CrossRef
[20] Garber A, Henry RR, Ratner R, Hale P, Chang CT, Bode B. Liraglutide, a once-daily human glucagon-like peptide 1 analogue, provides sustained improvements in glycaemic control and weight for 2 years as monotherapy compared with glimepiride in patients with type 2 diabetes. Diabetes Obes Metab. 2011;13(4):348-56. PubMed PubMed Central CrossRef
[21] Pratley RE, Nauck M, Bailey T, et al. Liraglutide versus sitagliptin for patients with type 2 diabetes who did not have adequate glycaemic control with metformin: A 26-week, randomised, parallel-group, open-label trial. Lancet. 2010;375(9724):1447-56. PubMed CrossRef
[22] Jensen TM, Saha K, Steinberg WM. Is there a link between liraglutide and pancreatitis? A post hoc review of pooled and patient-level data from completed liraglutide type 2 diabetes clinical trials. Diabetes Care. 2015;38(6):1058-66. PubMed CrossRef
[23] Victoza. Product information. EMA [Internet], 2009 Accessed on February 13, 2016. Available from: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/001026/WC500050017.pdf
[24] Victoza. Prescribing information highlights. FDA [Internet], 2012. Accessed on June 27, 2012. Available from: http://www.accessdata.fda.gov/drugsatfda_docs/label/2012/022341s017lbl.pdf
[25] Yang W, Chen L, Ji Q, et al. Liraglutide provides similar glycaemic control as glimepiride (both in combination with metformin) and reduces body weight and systolic blood pressure in Asian population with type 2 diabetes from China, South Korea and India: A 16-week, randomized, double-blind, active control trial. Diabetes Obes Metab. 2011;13(1):81-8. PubMed CrossRef
[26] Zang L, Liu Y, Geng J, et al. Efficacy and safety of liraglutide versus sitagliptin, both in combination with metformin, in Chinese patients with type 2 diabetes: A 26-week, open-label, randomized, active comparator clinical trial. Diabetes Obes Metab. 2016;18(8):803-11. PubMed PubMed Central CrossRef
[27] Wangnoo SK, Kumar S, Bhattacharyya A, et al. Liraglutide effect and action in diabetes-In (LEAD-In): A prospective observational study assessing safety and effectiveness of liraglutide in patients with type 2 diabetes mellitus treated under routine clinical practice conditions in India. Indian J Endocrinol Metab. 2016;20(6):838-45. CrossRef
[28] Victoza. Product information. MIMS [Internet], 2011. Accessed on February 13, 2017. Available from: http://www.mims.com/philippines/drug/info/victoza?type=full
[29] UNITE for Diabetes Philippines. Philippine practice guidelines on the diagnosis and management of diabetes mellitus, 2014. Available from: http://obesity.org.ph/v4/wp-content/uploads/2014/07/Diabetes-United-for-Diabetes-Phil.pdf
[30] Gautier JF, Martinez L, Penfornis A, et al. Effectiveness and persistence with liraglutide among patients with type 2 diabetes in routine clinical practice--EVIDENCE: A prospective, 2-year follow-up, observational, post-marketing study. Adv Ther. 2015;32(9):838-53. PubMed PubMed Central CrossRef
[31] Buysschaert M, D'Hooge D, Preumont V, Roots Study G. ROOTS: A multicenter study in Belgium to evaluate the effectiveness and safety of liraglutide (Victoza®) in type 2 diabetic patients. Diabetes Metab Syndr. 2015;9(3):139-42. PubMed CrossRef
[32] DeVries JH, Bain SC, Rodbard HW, et al. Sequential intensification of metformin treatment in type 2 diabetes with liraglutide followed by randomized addition of basal insulin prompted by A1C targets. Diabetes Care. 2012;35(7):1446-54. PubMed PubMed Central CrossRef
[33] Pratley RE, Nauck MA, Bailey T, et al. Efficacy and safety of switching from the DPP-4 inhibitor sitagliptin to the human GLP-1 analog liraglutide after 52 weeks in metformin-treated patients with type 2 diabetes: A randomized, open-label trial. Diabetes Care. 2012;35(10):1986-93. PubMed PubMed Central CrossRef
[34] Drab SR. Glucagon-like peptide-1 receptor agonists for type 2 diabetes: A clinical update of safety and efficacy. Curr Diabetes Rev. 2016;12(4):403-13. PubMed PubMed Central
[35] Ostawal A, Mocevic E, Kragh N, Xu W. Clinical effectiveness of liraglutide in type 2 diabetes treatment in the real-world setting: A systematic literature review. Diabetes Ther. 2016;7(3):411-38.PubMed PubMed Central CrossRef
Authors are required to accomplish, sign and submit scanned copies of the JAFES Author Form consisting of: (1) Authorship Certification, that all the requirements for authorship have been met by each author, and that the final version of the manuscript has been read and approved by all authors; (2) the Author Declaration, that the article represents original material that is not being considered for publication or has not been published or accepted for publication elsewhere; (3) the Statement of Copyright Transfer [accepted manuscripts become the permanent property of the JAFES and are licensed with an Attribution-Share Alike-Non-Commercial Creative Commons License. Articles may be shared and adapted for non-commercial purposes as long as they are properly cited]; and the ICMJE form for Disclosure of Potential Conflicts of Interest. For original articles, authors are required to submit a scanned copy of the Ethics Review Approval of their research as well as registration in trial registries as appropriate. For manuscripts reporting data from studies involving animals, authors are required to submit a scanned copy of the Institutional Animal Care and Use Committee approval. For Case Reports or Series, and Images in Endocrinology, consent forms, are required for the publication of information about patients; otherwise, appropriate ethical clearance has been obtained from the institutional review board. Articles and any other material published in the JAFES represent the work of the author(s) and should not be construed to reflect the opinions of the Editors or the Publisher.