Landmark trials established benefits of glycemic control but forewarned against severe hypoglycemia.[1] Associated factors include those on insulin, sulfonylurea, or polypharmacy; with antecedent hypoglycemia, increasing age, prolonged diabetes, menopause, neurologic, renal, cardiovascular, or hepatic diseases. Hazards include dementia, fall, cardiovascular events, poor quality of life, IHA, further hypoglycemia and mortality. Akin to hypoglycemia is IHA, forming a vicious cycle.[2] IHA is defined as the onset of hypoglycemia before autonomic warning symptoms, presents as inability to perceive hypoglycemic symptoms, and is linked to hypoglycemia-associated autonomic failure.[3] Clamp studies demonstrated lower magnitude of glucagon and epinephrine responses even when glucose dropped to 59 mg/dL among elderly patients with diabetes.[4] At blood glucose below 50 mg/dL on a day post-hypoglycemia, epinephrine release was lowered to 200 ng/L which was half that observed when with prior hyperglycemia.[5] Brain lactate, the alternative fuel source, decreased among patients with Type 1 diabetes with IHA whereas those without showed stable lactate.[6] Thalamic blood flow increase that positively correlated with epinephrine response observed during moderate hypoglycemia.[7] IHA prevalence approaches 10% among T2DM patients on insulin, and 6% among those on oral hypoglycemic agents.[8] IHA prevention starts with detection. Current methods of symptom reporting and monitoring are either costly or irrelevant to symptoms hence the role of symptom-dependent questionnaires. Defining hypoglycemia remains challenging because thresholds for symptoms decrease with hypoglycemia, but increase with worsening diabetes. Currently recommended for IHA and recurrent hypoglycemia, CGM detects IHA with 88% specificity and 75% sensitivity.[9]-[10] Among 4 IHA questionnaires published, namely GS (Gold Score), CHI (Clarke Hypoglycemia Index), PBQ (Pederson-Bjergaard Questionnaire), Hypo-AQ (Hypoglycemia Awareness Questionaire), only the single-question Likert scale GS and the eight-item questionnaire CHI were validated.[11],[12],[13],[14] A comparison study among GS, CHI, and PBQ demonstrated hypoglycemia at 24%, 26%, and 62% respectively, with only the first two approximating prevalence studies at 25%.[15] Whereas CHI has Spanish and Catalan versions recently validated, there is no Filipino questionnaire.[16]
This study aimed to develop a validated questionnaire on IHA adapted from CHI by: (1) formulating a culturally-adapted Filipino version of CHI; (2) evaluating content validity of the translated and modified CHI; (3) the criterion validity against CGM with activity diary and receiver operator curve; (4) appraising the internal consistency.
METHODOLOGYThe first phase: Questionnaire Construction Phase, a questionnaire development study consisted of a linguistic translation of CHI and modification by: (1) literature review, (2) focused group discussions, (3) synthesis by an expert panel. The second phase: Questionnaire Administration Phase was a cross-sectional analytic study with: (1) pre-testing, (2) construct validity, (3) internal reliability, and (4) criterion validity test. It involved adult Filipinos with physician-diagnosed T2DM, based on American Diabetes Association Standards of Medical Care 2017 (Table 1), voluntarily recruited by active recruitment, gathered by purposive sampling from the Out-Patient Department of Philippine General Hospital. Patients were: Filipino by ethnicity, nationality, and residence; with physician-diagnosed T2DM, age 19 years and above, with informed consent, and any of the following: long-standing diabetes mellitus, on insulin or sulfonylurea, co-morbidities, and antecedent hypoglycemia. Patients unable to read and write, with retinopathy, cerebrovascular disease deficits, fractures, or amputations, were still included provided they had caregivers to assist them with the questionnaire. Patients who were pregnant, or with psychiatric comorbidities, or who did not give their consent were not included in the study. For Phase I Part 2, Questionnaire Modification Phase, a focused group discussion with 6-8 study participants per group, and a total of 4 groups were conducted.[17] For Phase II Part 1 or Pre-testing, 10% of the final testing sample size or a group of 8 patients participated.[18] For Phase II Part 2 Criterion Validity, using the prevalence of 6%, confidence interval of 95%, maximum margin of error of 7%, and preset sensitivity of 90%, the calculated sample size of 68 T2DM patients were recruited.[19]For Phase 2 Part 4 Construct Validity, 108 patients were recruited, tthe sample size to account for subject-to-item exploratory factor analysis ratio of 10 respondents for every 1 item question, that is minimum 80.[18]
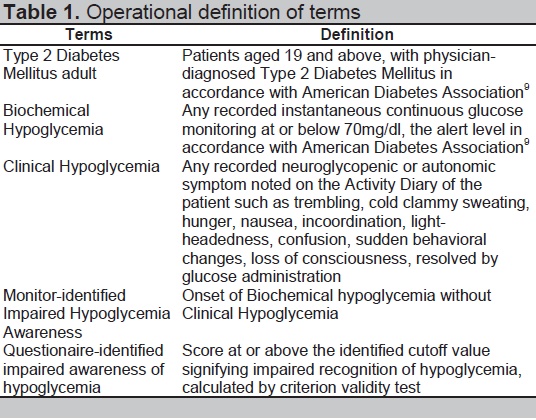
Table 1. Operational definition of terms
Phase I: Questionnaire Construction
Phase I Part I: Translation, Backward Translation and Synthesis
Komisyon ng Wikang Filipino translated the Clarke Hypoglycemia Index into Filipino. Sentro ng Wikang Filipino independently translated the Filipino version back into English. Forward and backward translations were collated by an expert panel of: (1) adult endocrinologist, (2) public health doctor, (3) the previous forward and back translators, who formulated the Translated CHI version.
Phase I Part 2: Modification
Phase I Part 2a: Literature Review and Focused Group Discussion (FGD)
Literature search from PUBMED, HERDIN, and Google Scholar was used then incorporated into concept list. There were 4 Focus Group Discussions (FGD) held, each with 6-8 patients. Translated CHI was modified using the concept list and FGD into a concept map (Figure 1), as recommended by expert panel of doctors specialized in: (1) adult endocrinology, (2) internal medicine, (3) neurosciences, and (4) public health. The output was Modified CHI.
Phase I Part 2b: Content Validity
Modified CHI was graded by Content Validity Scores (CVS), a Likert scale of 1 to 4 with: 1- least relevant and must be removed, 2- must modify entire statement to be included, 3- must modify a word to be included, or 4- most relevant and must be included. A CVS of at least 83% qualified it for inclusion.[20] The output was Filipino-CHI draft.
Phase 2: Questionnaire Administration
Phase 2 Part 1: Pretesting
Filipino-CHI draft was pretested on 9 adult Filipino T2DM patients interviewed regarding clarity, acceptability, and tolerability of the instructions and questions. Length of time taken was recorded. The output was Pre-tested Filipino-CHI.
Phase 2 Part 2: Criterion Validity Test against CGM
Pre-tested Filipino-CHI was compared with the hypoglycemic events noted on a CGM not recorded as symptomatic on the patient’s activity diary. Patients were admitted in the wards for 24-hour observation wearing the validated iPro2 CGM. Patients recorded activities, meals, exercise, autonomic or neuroglycopenic symptoms in the Activity Dairy. CGM data, calibrated to validated capillary blood glucose, was uploaded to the iPRO2 software, and was correlated with Activity Diary. Biochemical Hypoglycemic Events (BHE) was defined as instantaneous glucose monitoring below 70 mg/dl in concordance to the consensus of ADA and Endocrine Society. Clinical Hypoglycemic Event (CHE) was defined as at least one hypoglycemic autonomic or neuroglycopenic symptom noted on Activity Diary (Table 1). Any BHE without concurrent CHE was considered as Monitor-identified IHA (mIHA). This was to differentiate it from the Questionnaire-identified IHA (qIHA) or the number of questions signifying IHA in the Pre-tested Filipino CHI. Calculations were done by: (1) arbitrarily setting cutoffs for qIHA scores compared against mIHA scores; (2) plotting 1-Specificity against Sensitivity for every set cutoff; (3) depicting a resulting Receiver Operator Curve; (4) identifying the topmost intersecting point value representing the Pre-tested Filipino CHI cutoff for IHA.
Phase II Part 3: Internal Consistency Test
Pre-tested Filipino CHI was answered by 108 patients to assess Cronbach’s alpha coefficient of internal consistency.
Data analysis was done by an independent analyst separately for each corresponding phase. Study data was encoded in Microsoft Excel then was analyzed using Stata SE v.13 software. University of the Philippines Manila Research Ethics Board Panel approved the research. Developers of the CHI provided written approval for study.
Phase I: Questionnaire Construction Output
Phase I Part 1: Translation, Backward Translation and Synthesis Output
Forward translation was done by Komisyon ng Wikang Filipino. Back translation was done by Sentro ng Wikang Filipino. Both translations were assessed faithful to the original version, except for minor correctable grammatical errors.
Phase I Part 2: Modification Output
Literature search in PUBMED using keywords as follows: "hypoglycemia" [MeSH Terms] OR Hypoglycemia [Text Word] AND questionnaire AND unawareness yielded 103 hits, from which questionnaires GS, CHI and its Spanish/Catalan translations, PBQ, Hypo-AQ were gathered. For local data, a search in HERDIN using keyword “hypoglycemia,” local publication type, dating 1990-2015, resulted in 4 hits, none of which showed a questionnaire on impaired hypoglycemia awareness in Filipino.
Focused group discussions noted that adult Filipino diabetic patients differed from other patients, mostly in the lack of comprehension of hypoglycemia and IHA, necessitating a two-step explanation to introduce IHA. Elaboration of symptomatology and inciting events served as cues to help patients recall IHA.
IHA literature list and IHA discussion list generated a concept map (Figure 1) from which the final modified CHI-Filipino version was formulated. Concept domains identified included: (1) Elusive Euglycemia model, (2) Developmental Model, and (3) Cognitive Models. The first model pertained to patients’ issues of being anxious about inability to consistently reach the ideal glucose level which cause patient maladaptation practices risking hypoglycemia and IHA. This was represented by Question Number 4 which related to fear of hypoglycemia; and Question Number 8 which related to patient-identified change or reduction of sensitivity to his/her hypoglycemia episodes. Developmental model pertained to exposures predisposing to the occurrence of IHA. This was represented by Questions Number 2, 3, 5 evaluating severe hypoglycemia episodes and symptomatic hypoglycemia. Cognitive model pertained to presence of patient’s baseline ability to sense hypoglycemia as was represented by Questions Number 1, 6 and 7. The significant domain was hypoglycemia, a cause and effect factor of IHA, and a cause of all 3 other domains, hence separately connected to impaired hypoglycemia awareness, and all three models. Subheadings of each domain were represented by the introductory paragraph elaborating on hypoglycemia preceding the IHA questions.
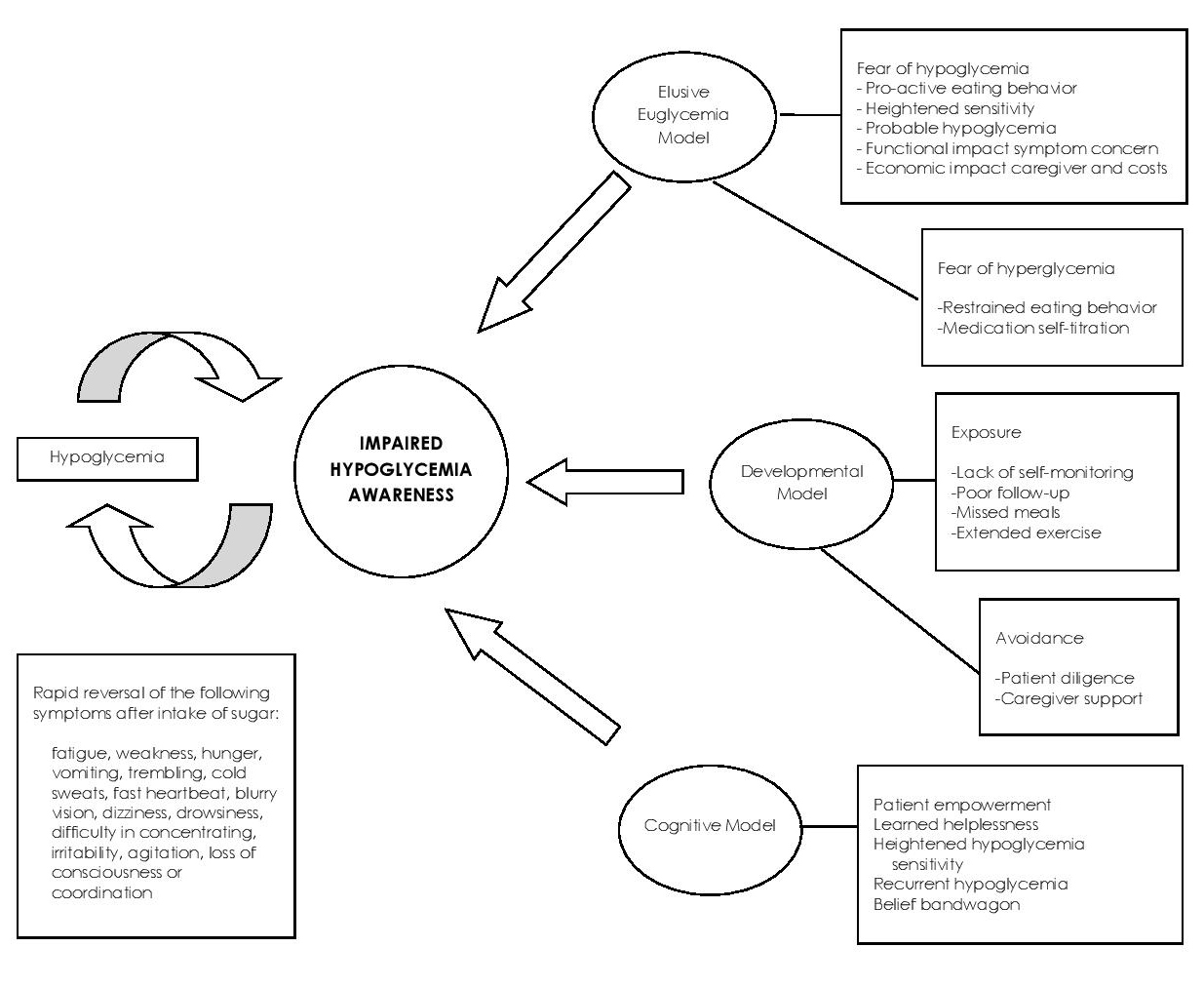
Figure 1. Concept map (English) of the Filipino version of the Clarke Hypoglycemia Index.
Questionnaire was evaluated for Content Validity Scores showing: Questions Number 1-6 with 93.75%, Questions Number 7 and 8 with 87.5% (Table 2).

Table 2. Content validity scores of item questions of Filipino-Chi
Phase II: Questionnaire Administration Output
Phase II Part 1: Pilot testing Output
Pilot testing on 9 participants identified recommendations for: (1) change of “instruksyon” to “panuto”; (2) use of complete phrases in choices; (3) removal of glucose units “mg/dL”; (4) addition of choice “hindi naitala ang asukal ko sa dugo”; (5) inclusion of checkmark. Length of time to finish the questionnaire remained acceptable ranging from 2 to 21 minutes, with a median of 6 minutes. This could be attributed to the patients’ tendencies to forget the first half of the relatively longer questions, such as Questions Number 2 and Number 3, necessitating rereading. The output was Filipino-CHI Pretested.
Phase II Part 2: Criterion Validity Output
Criterion validity was assessed among 69 participants (Table 3). Congruence between CGM and Activity Diary among 69 patients yielded 21 patients or 30.4% with Biochemical Hypoglycemic Events (BHE), among which only 2 patients had Clinical Hypoglycemic Events (CHE) corresponding to intact hypoglycemia awareness or negative Monitor-identfiied IHA (mIHA). The remaining 19 patients or 90.4% had asymptomaic or positive mIHA.
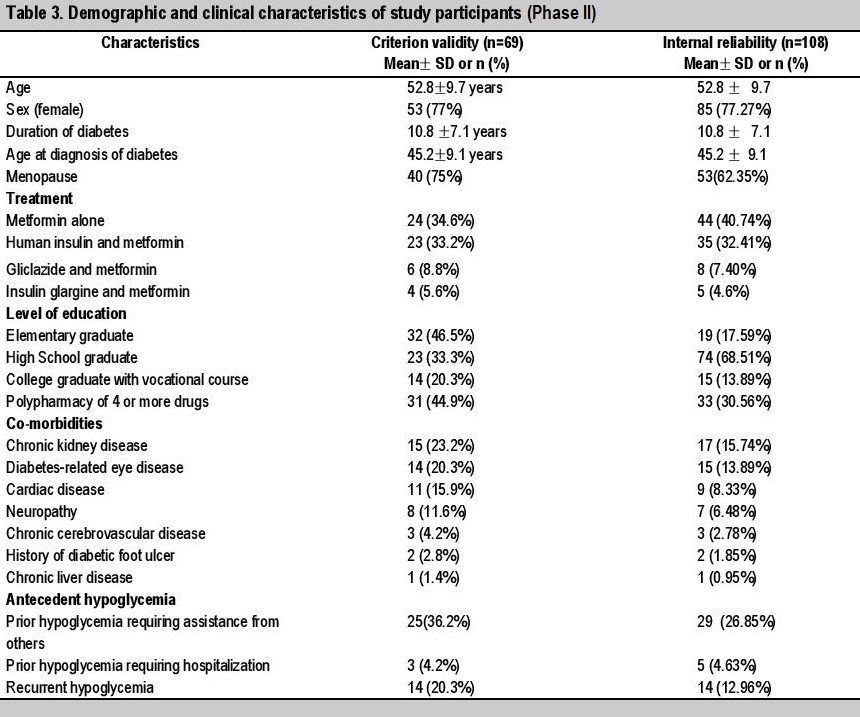
Table 3. Demographic and clinical characteristics of study participants (Phase II)
Questionnaire-identified IHA which corresponds to the performance of the Filipino-CHI questionnaire in detecting impaired hypoglycemia awareness was assessed by calculating sensitivity, specificity, positive predictive value, negative predictive value of each item question and of the entire questionnaire (Table 4). Adjustments for the Filipio-CHI questionnaire cutoff score defining impaired hypoglycemia awareness were done to identify the optimal cutoff scores. Setting questionnaire-identified IHA (qIHA) scores 4 yields an acceptable sensitivity of 89.47%, but specificity could not be properly calculated, owing to only 2 patients with negative mIHA, both of which barely scored 4/8 hence incorrectly labeled qIHA+ by the Filipino-CHI questionnaire. Setting qIHA scores 5 improved the Specificity to 100%, because then these 2 patients with negative mIHA become correctly labeled qIHA- by the questionnaire in the study, but at the expense of sensitivity diminishing to 26.31%. In the setting of only 21 hypoglycemia patients and 2 negative mIHA patients, the calculated area under the curve was 0.55.
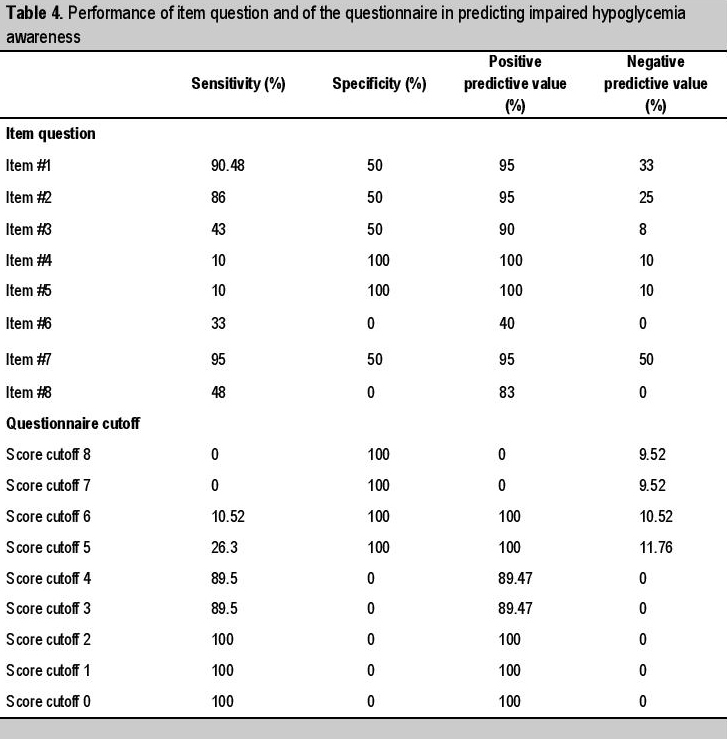
Table 4. Performance of item question and of the questionnaire in predicting impaired hypoglycemia awareness
Phase II Part 3: Internal Consistency Output
Internal consistency was assessed among 108 particpants (Table 3), none previously participating on the pilot testing, by calculating Cronbach’s alpha. Among them, 77.27% were females, 62.35% of whom were post-menopausal. The mean present age was 52.89 years old, mean age at diagnosis of diabetes is 45.29 years old, and the mean duration of diabetes is 10.8 ± 7.1 years. Among them, more than half completed secondary educational level, 17.59% completed elementary educational level, and the remaining 13.89% completed college or vocational degrees. Among them, 40.74% were on metformin alone, 32.4% were treated with human insulin and metformin, 7.4% with gliclazide and metformin. Among them, 30.56% were on polypharmacy of 4 or more drugs, 15.74% had nephrologist-diagnosed chronic kidney disease, 13.89% had ophthalmologist-diagnosed diabetic retinopathy or non-traumatic cataract. Among them, 26.85% had prior hypoglycemic episodes requiring assistance from others, 12.76% had recurrent hypoglycemia, and 4.63% had prior severe hypoglycemia requiring hospital management.
With only 8 questions subdivided into only 3 domains answered by this particular group of 108 participants, Cronbach’s alpha of the entire questionnaire was 0.45, Elusive Euglycemia Model domain 0.17, Developmental Model domain 0.39, Cognitive Model domain 0.33.
An eight-item Filipino-CHI questionnaire was formulated tailored to adult Filipino T2DM patients by literature review, four focus group discussions with patients and their caregivers, and consultations with relevant specialists who altogether graded each finalized item question with acceptable content validity scores. Gold Score (GS), the first internationally developed, is a validated visual analogue scale with the single question “Do you know when your hypos are commencing?” to be answered in a Likert scale of 1 to 7, where scores above 4 are labeled IHA. This single question is represented as Question number 1 in CHI and, Spanish and Catalan versionsof CHI, and this Filipino-CHI questionnaire. Similar to the original, Spanish and Catalan versions of CHI, the Filipino-CHI had impaired hypoglycemia awareness cutoff score optimally set at ≥4 answers that correspond to “R or reduced awareness”. Contrary to other questionnaires evaluating hypoglycemia awareness, this Filipino-CHI questionnaire was the only tool which underwent criterion validity testing against a measurable biochemical variable, the continuous glucose monitoring. This is crucial because hypoglycemia awareness depends on baseline knowledge and symptom recognition hence making it a highly subjective concept with considerable inter-individual differences in perception. A cutoff level of R≥4 gives an acceptable screening tool performance with a sensitivity of 89.47%. The area under the curve (AUC) of 0.55 could not be properly assessed due to the limited percentage of patients experiencing hypoglycemia that is only 21 out of 69, of which only 2 out of 21 tested negative monitor-identified IHA could be used for calculating specificity and AUC. Still this is relatively higher than reportted IHA prevalence as a result of the inclusion criterion of IHA associated factors, informed monitoring, regulated diet, activity, compliance, and sleep of participants. BHE cutoff set at hypoglycemia safety alert level 70mg/dL instead of hypoglycemia symptom occurrence level 55 mg/dL may also have prematurely labeled some patients as mIHA.[22]
In assessing its internal consistency, Cronbach’s alpha coefficient yielded unfavorable results, owing to the minimal number of item questions and domains into which the concise concept of impaired hypoglycemia awareness was categorized. Covering all the impaired hypoglycemia issues accumulated in the concept map, only 8 item questions about IHA were generated. The concept map illustrated the broadness of hypoglycemia concept and the conciseness of hypoglycemia awareness itself. This was reflected in the finalized questionnaire with a preceding comprehensive description of the concept of hypoglycemia, followed by the succinct eight-item interrogation about hypoglycemia awareness. For this reason, content validity test and criterion validity test may be more relevant than internal reliability, because the latter improves with increasing number of concept domains and item questions. Cronbach’s alpha is lowered by brevity of the test, because it is improved by each related item question testing the same concept.[22] More importantly, Cronbach’s alpha is a function of the scores on a test from that specific group of participants, for which it is recommended not to depend on published alpha estimates and is advised to measure alpha each time a test is utilized. Because this study was conducted in the service out-patient clinics of a government-subsidized tertiary referral center, the participants involved were mostly aged, females, with long-standing T2DM, and notably non-working attaining secondary level education only.
Prevalence studies among Filipino T2DM patients revealed characteristics similar in being mostly aged 61.56 ± 11.3 years old, females at 68%, long-standing 9 ± 6 years, but strikingly dissimilar in educational attainment that is a greater percentage reaching college level in 50% than secondary level in 32%.[23] Because this questionnaire evaluated impaired hypoglycemia awareness, sensitivity to and ability to report hypoglycaemia necessitates adequate knowledge, understanding, memory and logic which may be improved with increasing educational attainment. This questionnaire may hence perform differently among patients with diabetes mellitus in the community. Similarly, factor analysis using Kaiser criterion or scree plot was not performed because of the initial brevity of the questionnaire formulated. For this reason, the questionnaire validity tests performed might have been limited by having to tailor to this particular group of participants in this study. Further multi-center studies conducted on a bigger number of adult patients with T2DM whose characteristics better represent adult Filipinos with T2DM in a setting that better typifies their daily activities may yield a different result.
An 8-item questionnaire evaluating IHA tailored to adult Filipinos with T2DM was developed and validated with acceptable content validity and criterion validity, and may be used in screening for IHA who may then be recommended to have confirmatory CGM. Further multi-center studies conducted on a bigger number of adults with T2DM whose characteristics better represent adult Filipinos with T2DM in a setting that better typifies their daily activities may yield a different result. Validation on population with higher risk for IHA such as patients with Type 1 Diabetes Mellitus may be a focus of future studies.
Statement of AuthorshipAll authors certified fulfillment of ICMJE authorship criteria.
Author DisclosureThe authors declared no conflict of interest.
Funding SourceThis study received a research grant from the Philippine Society of Endocrinology, Diabetes, and Metabolism. Two machines were donated: Ipro2 continuous glucose monitor and contour capillary blood glucose monitor.
[1] Intensive blood-glucose control with sulfonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). UK Prospective Diabetes Study (UKPDS) Group. Lancet.1998;352(9131):837-53. PubMed.