Allergy to insulin has become rare with the advent of human insulin and its analogues. The incidence of insulin allergy is less than 1% in patients with diabetes. The diagnosis is based on clinical presentation and supported by skin or serological testing if available.
Insulin preparation requires additives such as protamine or zinc, which may act as a potential allergen. Therefore, allergy to insulin can be precipitated by the insulin molecule itself or carrier proteins.
Treatment options for insulin allergy include symptomatic therapy with antihistamines and use of alternative insulin preparation. Other therapeutic actions which have been reported include insulin desensitization using small doses of insulin or through a continuous subcutaneous insulin infusion (CSII), use of monoclonal antibodies (Omalizumab) and even pancreatic transplantation for severe, resistant cases.[1] We report a case of insulin allergy successfully treated using a modified insulin desensitization protocol.
A 27-year-old female with Type 2 Diabetes Mellitus (T2 DM) was initiated on insulin therapy for glucose optimisation for pre-pregnancy care. She was diagnosed with diabetes at the age of 25 years old and treated with Gliclazide (Diamicron MR ) 90 mg daily and Metformin 1 gram bid. She has poor glycaemic control with a recent HbA1c of 9.5%. There was no evidence of diabetes retinopathy, nephropathy or neuropathy.
Her initial insulin therapy included short-acting human insulin (Actrapid) and basal insulin (Detemir). She developed generalised urticarial rash 20 minutes after administration of short-acting insulin (Actrapid) and basal insulin (Detemir). She discontinued her insulin after developing similar reaction with subsequent insulin injections and resumed her oral antidiabetic agents. Her insulin regimen was switched to rapid acting insulin (Aspart) and basal insulin (Glargine) a month later during the clinic visit. Unfortunately, it resulted in anaphylactic shock, angioedema and bronchospasm requiring a hospital admission. She responded to rescue therapy with intravenous antihistamine, hydrocortisone and intramuscular adrenaline. While in the ward, trial with pre-mixed human insulin (Mixtard) resulted in bronchospasm which was relieved with intravenous hydrocortisone and antihistamine chlorpheniramine.
Further history revealed that she had previous allergies to gliclazide 80 mg tablet form, paracetamol and seafood. However, these allergies were confined to skin manifestation described as pruritus and urticarial rash. There was no similar history of allergy among her family members. None of her family members has asthma.
During the trial period of insulin initiation, she was concomitantly maintained on oral antidiabetic agents. After unsuccessful attempts to initiate patient on different types of insulin (insulin Actrapid, Insulatard, Mixtard, Aspart, Detemir and Glargine), she was referred to our centre for insulin desensitization therapy.
Initial blood test in our centre showed eosinophils count of 0.4 (reference range <0.4) with a total IgE of 78 kU/L (reference range <70). Unfortunately, skin prick testing or allergy testing for insulin was not available at our centre.
In view of the previous history of anaphylactic reaction, her insulin regimen was commenced at a very low dose of rapid-acting insulin (Glulisine). Rapid-acting insulin (Glulisine) was diluted to achieve a dose of 0.0001 IU. The initial 4 doses (0.0001, 0.0001, 0.001 and 0.005 IU) was given as an intradermal injection at a 30-minute interval. Pre-medication therapy included antihistamine loratadine. She developed transient erythema and itchiness at the site of injection which subsided within few minutes. There was no further development of urticaria. Subsequent insulin doses were administered subcutaneously at an incremental dose of 0.01 IU every 30 minutes, achieving a dose of 0.1 IU on Day 1.
After successful desensitization with a very low dose of rapid-acting insulin (Glulisine) on Day 1 and considering patient’s intention to conceive in near future, we switched her insulin to rapid-acting insulin (Lispro) as it has more safety data for use in pregnancy and better availability of rapid-acting insulin (Lispro) in the primary and secondary healthcare formulary near her hometown.
Desensitization therapy was continued using rapid-acting insulin (Lispro) at Day 2. We initiated low dose of rapid-acting insulin (Lispro) at 0.1 IU with an incremental dose of 0.05 IU every 30 minutes. Once her insulin dose reached a level of 0.5 IU, her incremental dose was given at 0.1 IU every hour achieving a maximum dose of 1 IU of insulin per injection. The incremental dose was given at 30 to 60 minute- intervals over a 12- hour duration for the first 2 days.
On day 3, rapid-acting insulin (Lispro) was given at 1 IU, 2 IU and 3 IU at 30- minute intervals. She was able to tolerate the slow increment of low insulin doses over the 3-day period without any allergic reactions.
On day 4, rapid-acting insulin (Lispro) was titrated to 4 IU tid and intermediate-acting human insulin (Insulatard) 10 IU was added at bedtime. A daily allowance of 12 IU of rapid-acting insulin (Lispro) and 10 IU of intermediate-acting insulin (Insulatard) combined with gliclazide (Diamicron MR) 90 mg od and metformin 1 g bid was achieved with blood sugar ranging between 7 to 13 mmol/L throughout the day.
The patient was referred back to her local clinic doctor with further titration of rapid-acting insulin (Lispro) and discontinuation of gliclazide (Diamicron MR) within 2 weeks.
Allergy to insulin is rare with a prevalence of 2% worldwide.1 Although most allergic reactions are local and confined to the site of injection, systemic reactions involving generalized urticarial rash and anaphylaxis have been reported. These allergens include insulin molecule or excipients such as preservatives (i.e., metacresol), retardants (i.e., protamine sulphate), stabilizers (i.e., zinc), acid and base buffers, and isotonic agents (i.e., glycerol).[1],[2]
The advent of analog insulin has reduced the incidence of insulin allergy. Allergenicity of insulin has been proposed by chemical changes in terminal B chains which have been modified in analog insulin.[1] It has been reported that the ability of analog insulin to reduce immunogenicity is associated with its rapid absorption rather than changes in the immunogenic epitopes itself.[1]
Treatment of insulin allergy includes antihistamine and use of alternative insulin preparation. Allergy to insulin excipients is more common than allergy to the insulin molecule itself. Treatment includes replacing it with insulin without the suspected allergenic excipients. However, this may be difficult.
Wheeler et al., found that metacresol is universally present in available commercial insulin.[2],[3] Metacresol is present in all insulin preparations (insulin Aspart, Detemir, Glargine, Actrapid, Insulatard, Mixtard, Lispro) tested on our patient, which suggests that it acts as a potential allergen. Previous reports have shown a dose-response relationship: the lowest reaction was seen with intermediate-acting Humulin NPH® (metacresol 1.6 mg/mL) and the most severe reactions were seen with rapid-acting insulin (Lispro and Glulisine) (metacresol 3.15 mg/mL).[2] A further intradermal test or specific IgE test would help to identify the allergen involved5 but these tests were not available in our centre.
Options for treatment of metacresol allergy are limited. Past insulin preparations which did not contain metacresol, such as porcine insulins Monotard®, and Ultratard® are no longer available. However, since metacresol is present as a preservative in almost all commercially available insulins, desensitisation protocol is a reasonable approach.
The most common type of insulin allergy is related to an IgE-mediated Type 1 allergic reaction of the Coombs and Fell classification.[2] Type III Arthus type reaction is less frequent. In addition, insulin hypersensitivity can be related to a T-cell mediated Type IV reaction. Desensitization is usually successful in IgE-mediated type 1 reaction, as in our case.[3],[4]
There is no standard protocol for insulin desensitization regimen. Insulin desensitization can be in the form of micro doses of insulin, subcutaneous continuous insulin infusion (SCII) or low basal rate of intravenous insulin infusion running between 0.1 IU/hour to 0.3 IU/hour.[1]
The mechanism for tolerability of intravenous insulin infusion is unclear. Suggested mechanism includes different responses of the immune system to the route of insulin administration.[2] A simple mechanical explanation is due to the rapid distribution of the relatively small volume of insulin into a larger central venous circulation.[2]
A report by Pfohler et al., recommended an ultra-rush protocol with subcutaneous insulin application (0.004, 0.01, 0.02, 0.03, 0.1, 0.2, 0.5 and 1.0) with an injection interval of 30 minutes achieving intended insulin dose of 12 units by Day 3 with a decreased local reaction in a T2 DM patient with insulin allergy.[3] Desensitisation protocol by R Barranco et al., included an initial insulin dose of 0.001 IU with a cumulative dose of 9 IU by Day 3 without any pre-treatment with antihistamine.[5] However, upon further increase of insulin doses to 15 IU tid, oral antihistamine was added for local urticarial reaction.[5] Most of the insulin desensitization protocol included oral antihistamine. All these patients who underwent insulin desensitization protocol as reported by Claudia et al., and R Barranco et al., experienced mild local reactions despite insulin initiation at a low dose of 0.001 IU.
In view of previous allergic and life-threatening reactions experienced by our patient, we modified the insulin desensitization protocol to start at a very low dose of analog insulin and frequent administration of very low doses of insulin, given as an intradermal injection to allow for a stable desensitization and avoiding any detrimental side-effects. An increase in 5 to 10-fold insulin concentration was given at subsequent doses at 30-minute intervals on day 1. No steroid coverage was given as there was no visible rash noted and no hemodynamic instability during the therapy. Dose titration continued if the allergic reaction was transient. Patient eventually developed tolerance to rapid-acting insulin lispro and intermediate-acting insulin NPH insulin after 72 hours of desensitization.
In comparison to previous reports, our modified desensitization protocol given at a very low dose of insulin and frequent incremental dose for a total of 12 hour duration was tolerable with no obvious adverse reactions seen in our patient (Table 1). The mechanism is unclear. The time required for successful desensitization with any protocol varies according to patient, technique used and the availability of alternative treatment.[6] No steroid was used as the insulin was administered at very low doses and at 30-min intervals to allow successful desensitization.
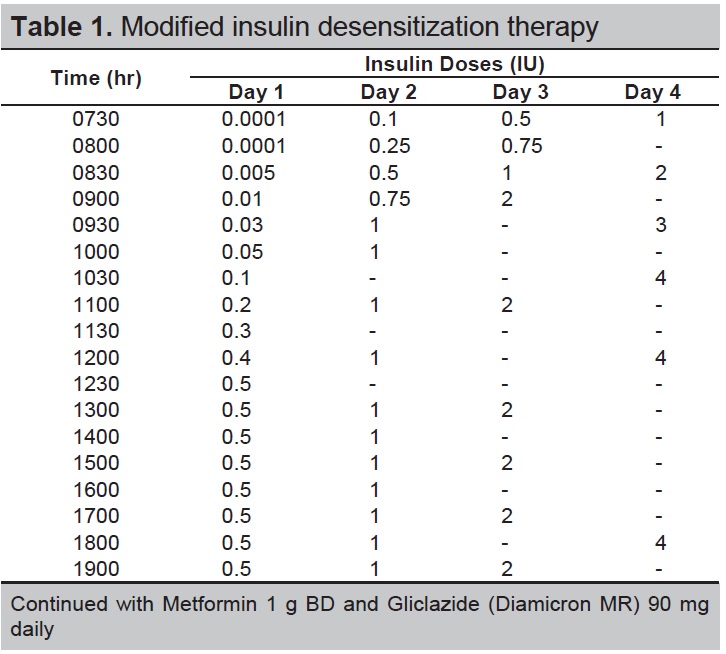
Table 1. Modified insulin desensitization therapy
Desensitization protocol in T1 DM patients with insulin allergy is more complicated as they require continuous insulin administration either via intravenous or subcutaneous infusion during the desensitization period in view of the state of absolute insulin deficiency.
The practicality and simple method of our modified desensitization protocol will be beneficial to T2 DM patients with insulin allergy.
There is no reported risk factors that can predispose patient to insulin allergy. Due to the rarity of insulin allergy and its excipients, it may not be feasible to conduct a controlled study of an insulin desensitization protocol.
Allergic reaction to insulin excipients which leads to systemic reactions such as anaphylaxis is rare. A modified desensitisation therapy proved to be successful in the management of allergy to insulin excipient in T2 DM patient.
Ethical ConsiderationAll means have been exhausted to obtain patient consent to no avail. All patient identifiers have been removed.
Statement of AuthorshipAll authors certified fulfillment of ICMJE authorship criteria.
Author DisclosureThe authors declared no conflict of interest.
Funding SourceNone.
[1] Matheu V, Perez E, Hernández M, et al. Insulin allergy and resistance successfully treated by desensitisation with aspart insulin. Clin Mol Allergy. 2005;3:16. PubMed PubMed Central CrossRef