Diabetic Foot Ulcer (DFU) is a common complication of Diabetes Mellitus (DM) that has increased dramatically over previous decades.[1],[2] The lifetime risk of a foot ulcer in patients with diabetes (type 1 or 2) may be as high as 25%.[3] DFU is one of the major causes of morbidity and mortality accounting for approximately two-thirds of all nontraumatic amputations performed in the United States.[3] In Malaysia, foot complications accounted for approximately 12% of all diabetic hospital admissions.[4] In Hospital Kuala Lumpur, which is the main public tertiary medical center in Malaysia, around 17% of diabetic patients were admitted because of diabetic foot ulcer (DFU).[5]
DFU is defined as a non- or poorly healing, partial or full thickness wound, located distal to the ankle in an individual with DM. The common sites involved are the sole of the foot or the toes.[6] Once DFU has developed, there is an increased risk of ulcer progression that may ultimately lead to amputation. Overall, the rate of lower limb amputation in patients with DM is 15 times higher than patients without diabetes.[7] Furthermore, DFU is responsible for substantial emotional and physical distress as well as productivity and financial losses that lower the quality of life.[8]
The primary management goal for DFU is to obtain wound closure as expeditiously as possible.[9],[10] However, glucose control measured by glycated hemoglobin (HbA1c) level is the most important metabolic factor.[11],[12] HbA1c level measures the average blood sugar concentration over a 90 day span of the average red blood cell in peripheral circulation. In the UKPDS, it was clearly shown that a 1% mean reduction in HbA1c is associated with a 25% reduction in microvascular complications, including neuropathy.[13] Poor glucose control accelerated the manifestation of peripheral arterial disease (PAD) which is a primary cause of DFU.[13] Meta-analysis of nine trials enrolling 19,234 patients showed that compared with less intensive glycemic control, intensive control (HbA1c, 6%-7.5%) was associated with a significant decrease in risk of amputation (relative risk [RR], 0.65; 95% confidence interval [CI], 0.45-0.94; I2= 0%).[14]
One of the retrospective studies demonstrated that single blood glucose level >12.2 mmol/L on the first postoperative day was a sensitive (87.5%) predictor of postoperative infection.[15] Recently, a retrospective study showed that there was a significant association between HbA1c variability and healing time in diabetic foot ulcers. Additionally, the study also highlighted that time to healing is more dependent on the mean HbA1c than the variability in HbA1c (p=0.007).[16]
However, to date, no prospective study has been performed to assess the effect of glycemic control to decrease HbA1c levels has benefits in wound healing after a foot ulcer has developed.[17] This is a pilot study conducted with the main objective of evaluating the association of HbA1c reduction and wound healing rate.
METHODOLOGYStudy design
A 12-week prospective, non-controlled, interventional study in subjects with suboptimally controlled T2DM patients with DFU was conducted from June to December 2016 at the Wound Unit, Hospital Putrajaya. The study was approved by the local institutional review board. Informed patient consent was obtained.
Study population
Majority of patients were referred by the Health Clinic from Wilayah Persekutuan Putrajaya, Malaysia to improve and optimise the risk factors for management of wound healing. The dedicated wound team consisted of 3 medical officers and 3 staff nurses. All wound treatments were performed for all patients with diabetic wounds according to the Standard Operation Procedure (SOP) of the wound clinic including removal of non-viable tissue, local dressing (antimicrobial dressings with silver), offloading with proper shoes and antibiotic treatment if infection was present. However, patients’ glycaemia were not monitored at the wound clinic. Their glycaemic control and cardiovascular risk factors were managed by their respective doctors from the health clinic or specialists at the hospital.
Study patients
Eligible T2DM patients with DFU aged 20 to 75 years old who had baseline HbA1c 1% higher than the target were recruited to the study. The target HbA1c was determined at the first visit based on the patient’s age, duration of DM, comorbid, diabetic complications, life expectancy and risks of hypoglycemia, according to Clinical Practice Guidelines – Management of Type 2 Diabetes Mellitus (5th edition).[18] Exclusion criteria were patients with acute and ongoing osteomyelitis or venous ulcer, patients with ankle brachial blood pressure ratio less than 0.5 suggesting severe limb ischemia, history (≥2 events) of hypoglycemic seizure or hypoglycemic coma within the last 6 months, patients with end stage renal disease, severe heart failure with New York Heart Association (NYHA) class IV, thromboembolic disease within the last 3 months, severe liver failure with Child–Pugh class C, history of schizophrenia, alcohol or drug abuse, and pregnant women. This study was approved by Malaysia Medical Research and Ethics Committee and was done in adherence to the Helsinki Guidelines. Written informed consent was taken during the first visit.
Study interventions
There were 5 visits during the study, i.e., week 0, week 2, week 4, week 8 and week 12. For each patient, demographic data, clinical data and laboratory biochemistry were collected including full blood count, renal function, liver function, HbA1c and lipid profile. Biochemical investigation was obtained during the first clinic visit and week 12 of the clinic visit. Renal function was evaluated by estimated glomerular filtration rate (eGFR) using Modification of Diet in Renal Disease formula. Detailed history and physical examination were performed.
Complication assessments including microvascular (retinopathy, nephropathy and neuropathy) and macrovascular (ischemic heart disease, stroke and peripheral vascular disease) were documented. During visits to the Wound Unit, blood pressure, pulse, and temperature were measured with the individual in a sitting position using standard clinical procedure. Associated cardiovascular risk factors including smoking, hypertension, dyslipidaemia as well as their treatment were ascertained.
Wound assessment
Detailed wound assessment was performed. Wound ulcer severity was graded by Wagner’s grading system from grade 1 to 5. Any presence of foot deformity, high plantar pressure, infections, inappropriate foot self- care, trauma, fracture, callus and amputation were documented. Serial wound areas, as determined by specific wound tracing grid at each visit, were indicative of the wound healing[19] (Figure 1). Initially, the wound tracing grid was put over the wound and area was traced on the grid. The superficial layer was peeled off and placed on a piece of paper. Subsequently, the wound area would be calculated using grid in cm2. The daily wound area healing rate in cm2 per day was calculated as the difference between wound area at the first visit and the subsequent visits, divided by the number of days between the two visits. In order to avoid measurement bias, an accurate measurement of the wound size was made by the same clinician by wound tracing and validated by a third independent clinician.
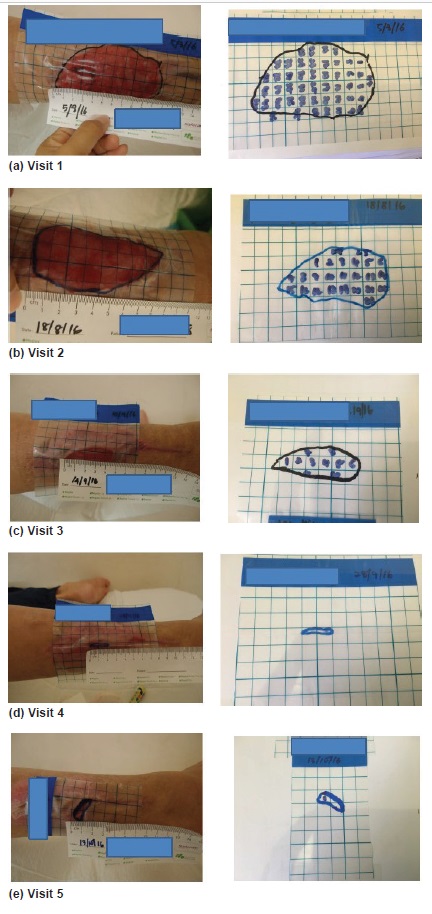
Figure 1. Measurement of wound area via wound area tracing at visits 1 to 5.
Individualised glycaemic intervention
The individualised glycaemic intervention was a continuous, integrated patient-centred care involving clinicians, staff nurses, nutritionists and diabetic educators. The areas emphasized included Medical Nutrition Therapy, Exercise Therapy, Diabetic Complications, Drug Adherence, Usage of Antidiabetic agents including Insulin Therapy, Hypoglycaemia Management, Self-Monitoring Blood Sugar (SMBG) with diary record, weight control, stress management, etc. We utilised social media and electronic devices as main communication tools in educating the patients.
Antidiabetic medications including insulin therapy or oral antidiabetic agents (OADs) were adjusted or added with the aim of reducing HbA1c by at least 1% in relation to patient’s individualised HbA1c target which was described above. Glucose meters and strips were given free to patients and SMBG with diary record was required at least 4 times per day (premeals and pre-bedtime) 3 times per week. Patients were encouraged to perform blood sugar checks whenever they were unwell or symptomatic for hypoglycemia. An ongoing insulin titration was performed actively in those patients whose glycaemia was not within the target range (including hypoglycemia) by the investigators or medical officers within the 12 weeks of their wound clinic visits. The titration of insulin was based on individualised titration protocol (based on Ministry of Health Clinical Practice Guidelines on Insulin Therapy).[18],[20] Antihypertensive and lipid lowering therapies were allowed to be added or adjusted depending on investigator’s discretion. The result of the glycemic intervention were expressed in absolute HbA1c reduction and relative HbA1c reduction rates calculated as the percentage of difference of first and final HbA1c divided by first HbA1c. Both were expressed in percentages (%).
Statistical analysis
All statistical analyses were performed using the Statistical Package for Social Science version 22.0 for Windows (SPSS Incorporation, Chicago, Illinois, USA). Descriptive analysis was used to explain demographic and clinical data. Numerical values for parametric and non-parametric variables were expressed as mean +/- standard deviation (SD) and median +/- Interquartile range (IQR). Categorical data were expressed as number and percentage. Spearman’s correlation was used to measure the strength and direction of association between the HbA1c reduction rate and the daily wound area healing rate. The patients were divided into 4 quartiles based on final HbA1c and the daily wound area healing rate for the first and fourth quartiles were compared via Wilcoxon signed rank test. A p value of < 0.05 was considered statistically significant.
Patient characteristics
There were 25 patients screened and recruited. Four patients had screening failure, including three who failed to meet the HbA1c criteria and two patients withdrew voluntarily from the study in the early period. Therefore, 19 patients (Table 1) completed the study. Their mean age and mean age of diagnosis of DM were 48.9 + 12.2 years and 37.1 + 9.5 years respectively. They were mainly males 15/19 (79%) and of Malay ethnicities 15/19 (79%). Their mean duration of DM and median duration of DFU were 10.8 + 6.7 years and 3 (2,6.5) months respectively. Macrovascular complications were present in 3 (16%) patients with the following distribution: ischemic heart disease 1 (5%), cerebrovascular disease (stroke) 2 (11%) and peripheral vascular disease with 3 (16%). All 19 patients (100%) had microvascular complications specifically retinopathy and neuropathy, and 14 (79%) patients had nephropathy. Forty eight percent and 26% of patients had target HbA1c of 6.5 – 7 % and 7-7.5% respectively.
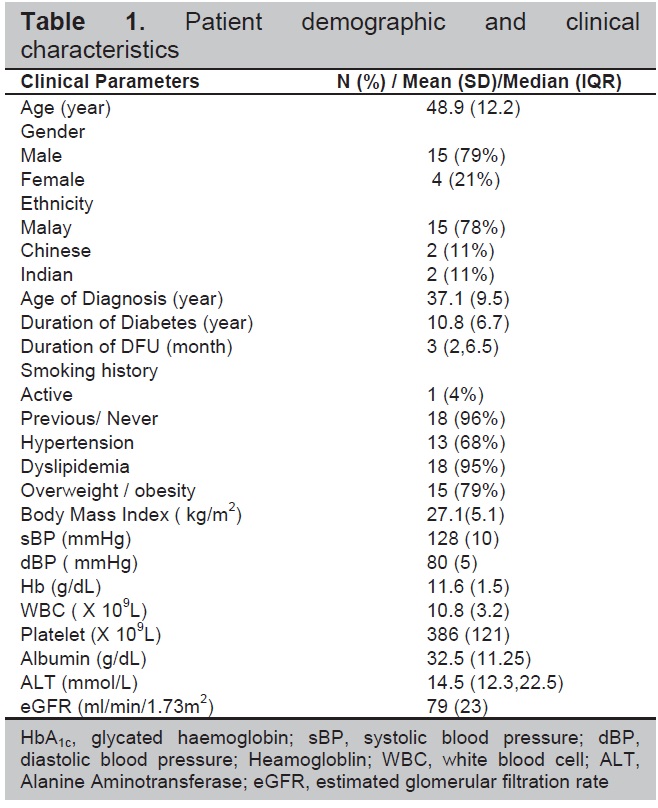
Table 1. Patient demographic and clinical characteristics
Antidiabetic agents
The patient’s baseline antidiabetic medications were mainly insulin (n=18, 95 %) and Metformin (n=12, 63%). The 3 main additional antidiabetic agents were Metformin (n=6, 32%), Dipeptidyl peptidase-4 inhibitor (DPP4i) (n= 4, 26%) and Sulphonylurea (n=2, 16%). During the first visit, 18 (95%) patients were on insulin therapy and 1 (5%) patient was on oral monotherapy. Among the patients on insulin, 11 (61%) were on combination of insulin therapy and one OAD. During the final visit, 2 (11%) patients were on two OADs and 17 (89%) patients were on insulin therapy. Among the patients on insulin, 12 (71%) were on combination with one OAD and 5 (29%) were on two OADs.
Wound characteristics
The median ankle-brachial pressure index for left lower limb and right lower limb was 1.07 (1,1.16) and 1.05 (0.95,1.13), respectively. Eighty four percent of patients had a single wound. Fifty three percent of patients had foot deformity or Charcot’s joint and inappropriate foot care. Seventy four percent of patients had Grade 2 or 3 ulcers according to Wagner’s classification of ulcer (Table 2).
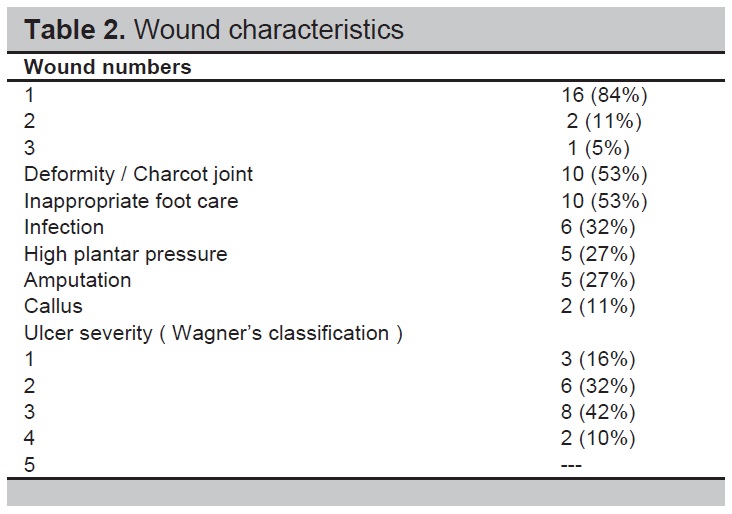
Table 2. Wound characteristics
Glycemic intervention and wound area healing rate
Eighteen (95%) patients had HbA1c reduction and 12 (63%) patients achieved the prespecified individualised target HbA1c. The mean HbA1c reduction rate was 31.2% + 7.5% and the median daily wound area healing rate was 0.234 (0.025, 0.453) cm2/day. There was a significant mean HbA1c reduction from 10.33 % + 1.83% to 6.89% + 1.4% (p<0.001) and mean total daily insulin reduction from 70.4IU + 19.6 IU to 41.6 IU +13.8IU (p<0.001). Spearman correlation analysis revealed that there was a strong positive correlation between the mean HbA1c reduction rate and median daily wound area healing rate. (r=0.752, p=0.01) (Figure 2). After dividing the patients into four quartiles based on final HbA1c and comparing the first quartile vs fourth quartile, there was a significant difference of daily wound area healing rate (0.597 vs 0.044 cm2/day, p=0.012). (Figure 3)
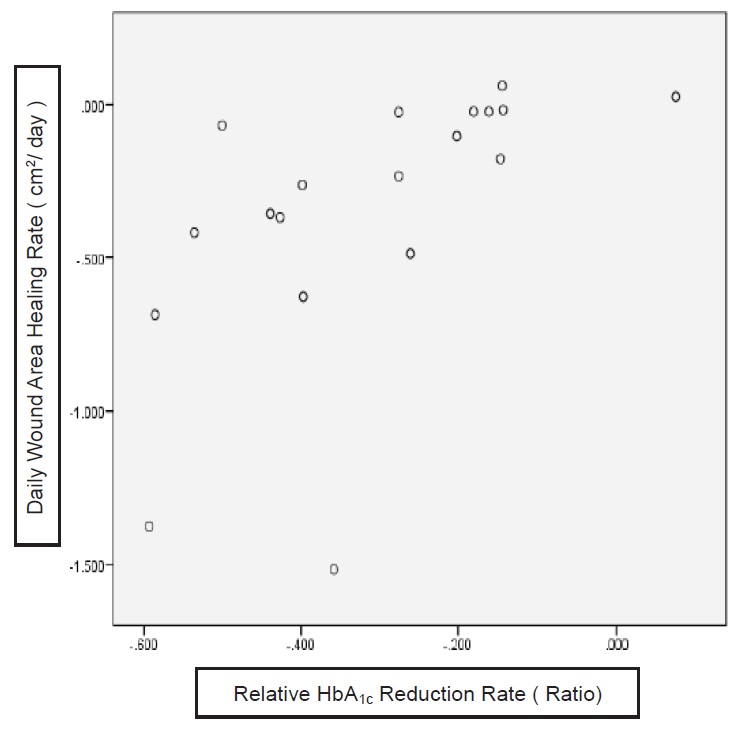
Figure 2. Correlation between the daily wound area healing rate and relative HbA1c Reduction Rate. Spearman correlation analysis revealed that there was a strong positive correlation between the two variables. (r=0.752, p=0.01).
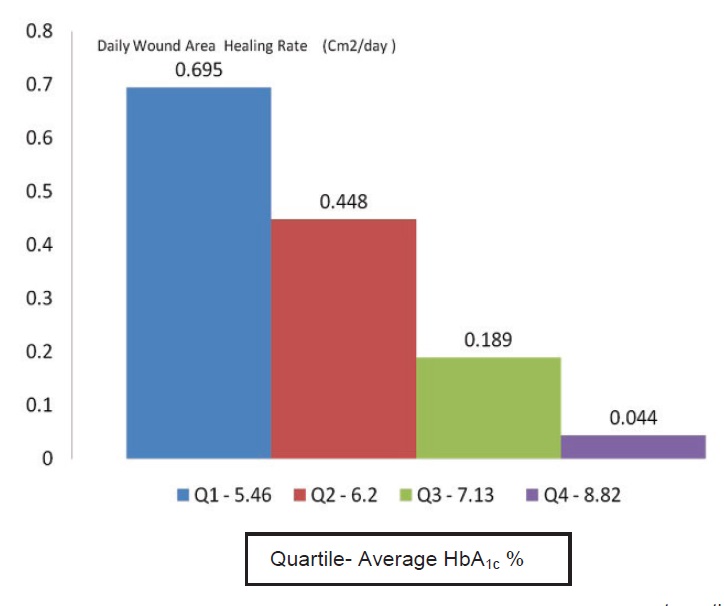
Figure 3. Daily wound area healing rate based on 1st - 4th quartiles of final HbA1c.
There was a significant positive correlation between HbA1c reduction rate and wound area healing rate. The lower the final HbA1c, the faster the wound healed. The higher reduction of HbA1c rate, the faster rate of wound healing compared to those who had lower HbA1c reduction rate.
In this 12-week study, 95% and 63% of patients had HbA1c reduction and achieved prespecified individualised target HbA1c respectively. The mean HbA1c reduction was 3.44 % and the HbA1c reduction rate was 33%. Yet the insulin requirement in our study was reduced by 41% accompanied by reduction of hypoglycemia events without any significant weight changes.
Their baseline antidiabetic medications were mainly insulin (95%) and metformin (63%). metformin (32%), DPP4i (26%) and sulphonylureas (16%) were the 3 main additional antidiabetic therapies during the study. Numerous randomized controlled trials and large observational studies have shown that insulin is the antidiabetic agent which has the greatest glucose lowering capacity, if compared with the other OADs.[13],[21] Therefore, insulin therapy was the major therapy to achieve the significant HbA1c reduction, albeit there were additions of new OADs. The only explanation of the reduction of insulin to achieve the HbA1c reduction was that the patients in the study had poor compliance to insulin therapy prior to the study.
The effective communication between patient and health care provider plays an essential part in managing patients with DFU. It provided adequate time and space and promote rapport, confidence, motivation and satisfaction for both parties. Individualised glycemic intervention in patients with DFU is a holistic tailored approach adapting to patient’s values, goal and preference, family, educational, socio-cultural, and occupational background. As diabetes is a multi-organ systemic disease, all comorbidities that affect wound healing must be managed by a multidisciplinary team to reduce amputation rates, lower costs, and lead to better quality of life.[22],[23],[24] Through structured education and self-management programmes, patients will be more adherent to the treatment, which have been shown to improve personal responsibility especially to their own medications.[25]
This is the pilot prospective study examining the association between individualised glycemic interventions and wound healing rate in patients diagnosed with DFU. The study was done in a specified wound clinic that allowed adequate facilities and expertise for patient care. There were certain limitations in the study including the small number of patients from a single center in a short duration of study period. Due to the reasons above, this study was designed as an uncontrolled study that would not provide a proper comparison of efficacy and safety of the intervention. The other limitation was the variable patients’ clinical conditions and wound characteristics with different wound treatment that might influence the results of the study. Ideally, a large number of patients from multiple centers including primary, secondary as well as tertiary health facilities should be examined to minimize the impact of the selection bias. Individualised glycaemic intervention should be integrated into multidisciplinary team approach in order to have better wound healing in patients with DFU.
There was a positive correlation between HbA1c reduction and wound healing rate in patients with DFU. Although this is an association study, the study postulated the benefits of achieving lower HbA1c on wound healing rate in DFU which require evidence from future randomised controlled studies.
AcknowledgmentsThe authors thank Dr. Siti Norlailli Aiza, Dr Nazirah and the staff nurses at the Wound Clinic (Sister Julizaayu, Sister Norita, SN Anita, SN Marliza), Hisham Abdullah (Diabetic Educator), Puan Norzalinah (Dietitian), Puan Nadiah (Statistician) and the Clinical Research Centre, Hospital Putrajaya for their contributions to this study.
Statement of AuthorshipAll authors certified fulfillment of ICMJE authorship criteria.
Author DisclosureAll the authors have declared no conflict of interest to the work carried out in this paper.
Funding SourceThe study was funded by Ministry of Health and Abbott Laboratories (M) Sdn. Bhd.
[1] Alavi A, Sibbald RG, Mayer D, et al. Diabetic foot ulcers: Part II. management. J Am Acad Dermatol. 2014;70(1):21.e1–2124. PubMed CrossRef