Seventy-Two Hour Mortality Prediction Model in Patients with Diabetic Ketoacidosis: A Retrospective Cohort Study
Nia Novianti Siregar,* Pradana Soewondo,** Imam Subekti,** Muhadi Muhadi***
Nia Nivianti Siregar, MD
Faculty of Medicine University of Indonesia
Jl. Salemba Raya No.4, RW.5, Kenari, Senen,
Kota Jakarta Pusat, Daerah Khusus Ibukota, Jakarta, Indonesia 10430
Tel. No.: +62-21-31930373
Fax No.: +62-21-3912477
E-mail: dr.nianovianti@gmail.com
ORCID:https://orcid.org/0000-0002-8207-0808
e-ISSN 2308-118x
Printed in the Philippines
Copyright © 2018 by the JAFES
Received April 7, 2018. Accepted May 31, 2018.
Published Online First: September 4, 2018.
Objective. This study aims to identify predictors of 72-hour mortality in patients with diabetic ketoacidosis (DKA).
Methodology. In this retrospective cohort study, data were obtained from medical records of adult patients with DKA in Cipto Mangunkusumo General Hospital from January 2011 to June 2017. Associations of predictors (age, type of diabetes, history of DKA, comorbidities, level of consciousness, renal function, bicarbonate, potassium, lactate, betahydroxybutyrate levels, and anion gap status) and 72-hour mortality were analyzed. The mortality prediction model was formulated by dividing the coefficient B by the standard error for all variables with p<0.05 in the multivariate analysis.
Results. Eighty-six of 301 patients did not survive 72 hours after hospital admission. Comorbidities (HR 2.407; 95% CI 1.181–4.907), level of consciousness (HR 10.345; 95% CI 4.860–22.019), history of DKA (HR 2.126; 95% CI 1.308–3.457), and lactate level (HR 5.585; 95% CI 2.966–10.519) were significant predictors from multivariate analysis and were submitted to the prediction model. The prediction model had good performance. Patients with total score less than 3 points were at 15.41 % risk of mortality, 3–4 points were 78.01% and 5–6 points were 98.22% risk of mortality.
Conclusion. The 72-hour mortality rate in Cipto Mangunkusumo General Hospital was 28.57%. The mortality prediction model had a good performance and consisted of comorbidities, history of DKA, level of consciousness and lactate level.
Keywords: prediction model, mortality, diabetic ketoacidosis
The incidence of diabetic ketoacidosis (DKA) as an acute complication of diabetes mellitus (DM) has been increasing with the increasing prevalence of DM.[1] DKA is characterized by the presence of hyperglycemia, acidosis, and a positive ketone test.2 DKA is a hyperglycemic crisis that can be fatal.[2] The mortality rate of DKA has been declining in developed countries (<10%) but increasing in developing countries.[3],[4] Previous study from Suwarto et al.,[5] in 2007-2008 showed that the five-day mortality rate of DKA patients in Cipto Mangunkusumo General Hospital was 40%.
Several previous studies showed different mortality predictors of DKA patients. Age and comorbidities were consistently found as significant mortality predictors in several studies.[6],[7],[8] Level of consciousness, type of DM, total dose of insulin used in DKA management and frequency of DKA episodes were also statistically significant mortality predictors in previous studies.[5],[9],[10],[11] Significant metabolic factors as DKA mortality predictors found in other studies were serum urea level, serum osmolarity, phosphate level, lactate level, renal function, and blood acidity.[6],[12],[13],[15],[16] Other laboratory parameters that are also impaired in DKA such as ketone, potassium and anion gap level have not been studied in relation to mortality in DKA patients.
Mortality of DKA patients generally occurs in the first 3 days of hospitalization, and it is recommended that DKA patients are admitted to an intensive care unit for the first 24–48 hours.[9] However, there are constraints due to limited intensive care unit capacity in Indonesia. Moreover, intensive care units are not evenly distributed throughout health facilities in Indonesia.
Given the seriousness of DKA, a prediction model is needed to assess the mortality risk of these patients within 72 hours of admission to hospital. Such a prediction model may provide a method for early stratification and prioritization of DKA patients with high mortality risk to receive treatment in an intensive care unit.
The aim of this study was to obtain a prediction model for 72-hour mortality risk in DKA patients. In addition, this study is also expected to provide information about 72-hour mortality rate of DKA patients in Cipto Mangunkusumo General Hospital and associated factors.
METHODOLOGYThis was a retrospective cohort study of DKA patients with all types of DM, aged ≥18 years, treated in the emergency unit of Cipto Mangunkusumo General Hospital, Jakarta, Indonesia, from January 2011 to June 2017. Data were obtained from medical and electronic health records in the hospital. Patients with incomplete or no medical records and those who were discharged against medical advice before 72 hours were excluded. DKA was diagnosed according to the following criteria: blood glucose >250 mg/dL, acidemia (pH <7.3 or bicarbonate level <18 mEq/L), and positive ketone test. Patients with high blood osmolarity level diagnosed as having hyperosmolar hyperglycemic state (HHS) were excluded. This study was approved by the Medical Ethics Committee of the Faculty of Medicine, University of Indonesia.
The outcome was defined as the 72-hour mortality of DKA patients. Eleven predictors were analyzed: age, comorbidities, history of DKA, level of consciousness, type of DM, and initial laboratory parameters including renal function (eGFR), potassium, bicarbonate, beta-hydroxybutyrate, lactate levels and anion gap status. All data are presented categorically. Laboratory tests included in analysis were results on admission. Beta-hydroxybutyrate was chosen because it is produced as much as two to three times versus acetoacetate and acetone in ketonemia. Using acetoacetate or acetone measurement only may lead to false negative results in the actual assessment of ketonemia. Comorbidities were defined according to the Charlson Comorbidity Index (CCI) and patients were divided into two groups: those with a CCI score of <5 (mild–moderate comorbidity) and those with a score of ≥5 (severe comorbidity).[17]
Minimum sample calculation to find the relationship between dependent and independent variables used calculations for logistic regression analysis with the formula "ten outcomes per variable" where the minimum number of samples is multiplication between the number of independent variables studied multiplied by 10 then divided by prevalence. From calculation and based on the mortality rate from previous study by Suwarto et al.,[5] minimum sample for this study was 275 subjects.
Data were analyzed using SPSS Statistics 20.0. Univariate analysis was used to identify associations between the outcome and predictors, and was performed by survival analysis with the log-rank test. The significance level used was α=0.05, and a significant association was defined as a p-value <0.05. Kaplan–Meier curve was used for survival analysis and followed by Cox proportional-hazard regression. All variables with p<0.25 in univariate analysis were included in the multivariate analysis. The cut off point p-value <0.25 in univariate analysis was chosen because more traditional levels such as 0.05 can fail in identifying variables known to be clinically important. In the iterative process of variable selection, covariates are removed from the model if they are non-significant (p-value >0.05) and not a confounder. The mortality prediction model was formulated by dividing the coefficient B by the standard error for all variables with p<0.05 in the multivariate analysis. Performance of the mortality prediction model was assessed using the receiver operating curve (ROC) and bootstrapping analysis using the Hosmer–Lemeshow calibration.
A total of 345 DKA patients aged ≥18 years were included. Fifteen patients were excluded because they had been discharged against medical advice, 4 patients because of incomplete medical records, and 25 patients because of no medical records found. Thus, a total of 301 patients were included in the analysis.
Patient characteristics are presented in Table 1. The most common precipitating factor was infection (57.1%), of which lung infection (45.3%) and DM ulcers (22.7%) were most common. Comorbidities recorded in this study were coronary heart disease 15.28%, cerebrovascular disease 11.96%, heart failure 10.29%, gastrointestinal bleeding 8.31%, malignancy 4.98%, chronic renal failure on dialysis 3.65%, and others 3.99%.
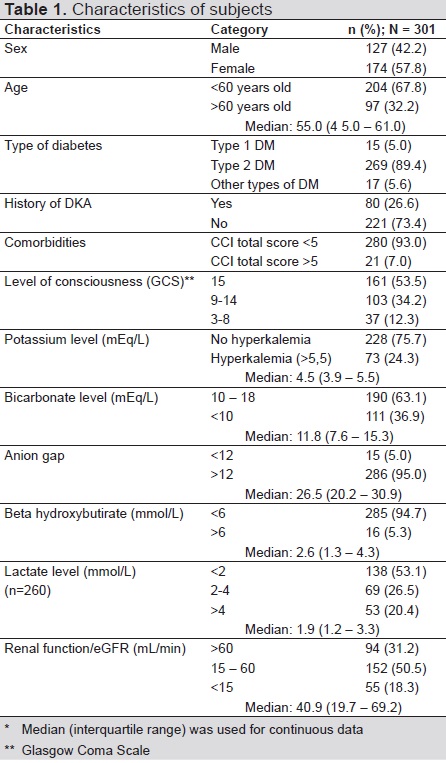
Table 1. Characteristics of subjects
The mortality rate within 72 hours was 28.57%. Six predictors were significantly associated with the 72-hour mortality rate in the univariate analysis: age (p=0.011), history of DKA (p<0.001), comorbidities (p<0.001), level of consciousness (p<0.001), renal function (p=0.018), and lactate level (p<0.001). These six predictors and three others with a p-value <0.25 in the univariate analysis were included in the multivariate analysis: type of DM (p=0.104), bicarbonate level (p=0.076), and anion gap status (p=0.144). The final step of the multivariate analysis is presented in Table 2.

Table 2. Multivariate analysis of predictors of 72 hours mortality
A mortality prediction model was formulated by dividing the coefficient B by the standard error for all variables with a p-value <0.05 in multivariate analysis. The final prediction model of 72-hour mortality in DKA patients is presented in Table 3. The prediction model was analyzed according to the ROC curve (Figure 1) and presented area under the curve (AUC) value of 0.893 (95% CI 0.856–0.929).
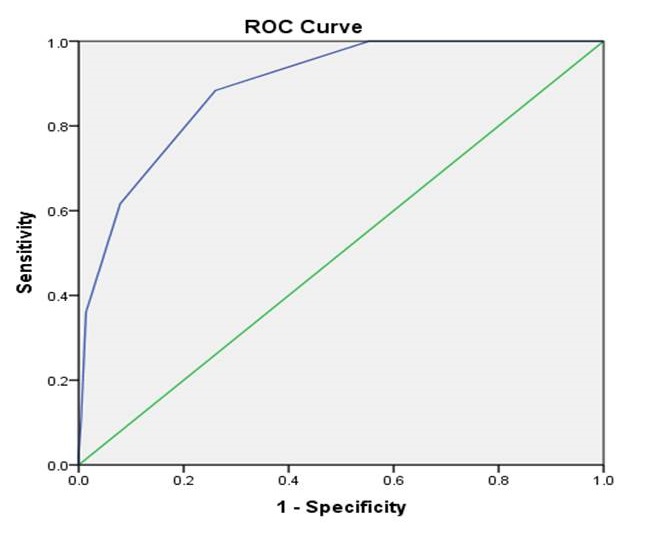
Figure 1. Area Under Curve (AUC) of 72 h mortality prediction model after Receiving Operating Curve
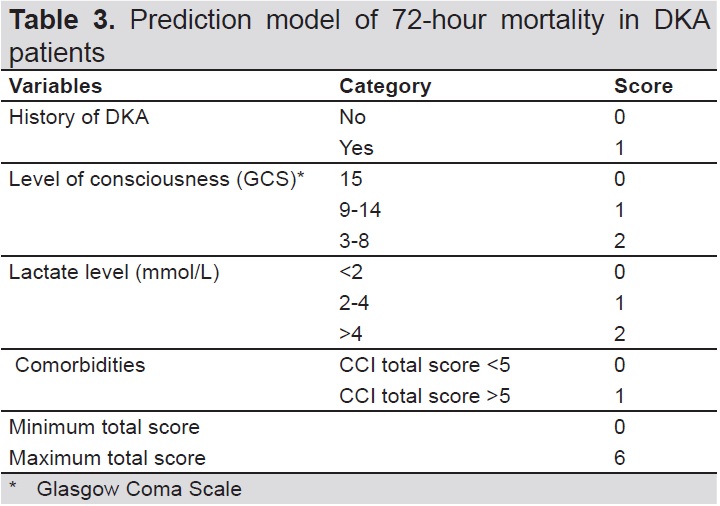
Table 3. Prediction model of 72-hour moratlity in DKA patients
Based on the cut off points for sensitivity and specificity, in the mortality prediction model based on the total score, the best cutoff point for estimating the 72-hour mortality of DKA patients was 2. Tables 4 and 5 present the probability of death in the first 72 hours for DKA patients based on 0–6 points for the total score in the prediction model. From table 5, a score of >2 predicts a significantly higher mortality than a score of 2 or less.

Table 4. The prognostic rule based on patients having zero to six points of total score from mortality prediction model

Table 5. Performance of integer-based mortality prediction model
A calibration test was performed for the mortality prediction model with a p-value of 0.208 before and after 1000 bootstrap samples. The prediction model showed good quality and strong discrimination.
Diabetic Ketoacidosis (DKA) is an acute complication of DM that is characterized by hyperglycemia, ketosis, and acidosis. It often accompanies type 1 DM, although patients with type 2 DM may also experience DKA as a result of catabolic stress associated with acute illness.[18] A study in the United States showed DKA can accompany metabolic disorders caused by absolute or relative insulin deficiency and increased counterregulatory hormones produced in response to precipitating factors such as infection, myocardial infarction, stroke, pancreatitis, or trauma.[19] Several medications such as corticosteroids can affect carbohydrate metabolism, which can trigger DKA.[3] Insulin withdrawal is the predominant precipitating factor for DKA in the United States.[2] This is different from the situation in Indonesia, where the main precipitating factor for DKA is infection (78.5–93%).[20] It is important to identify the precipitating factors as part of DKA management and prevention of recurrence of DKA.
DKA represents a hyperglycemic crisis that can be fatal.[2] Several studies have found that the mortality rate of DKA has declined during the past 20 years.[3] A study in United States found that the overall mortality from DKA in adult patients was <1%. The mortality rate for DKA was reported as >5% in elderly patients and in those with other life-threatening illnesses.[4] Moreover, the mortality rate of DKA remains high in developing countries.[3] Suwarto et al.,[5] reported in 2007–2008 that the mortality rate for DKA patients within 5 days of admission to Cipto Mangunkusumo General Hospital was as high as 40%.
Efstathiou et al.,[9] reported that death caused by DKA occurs mainly during the first 3 days of hospital admission and suggested that patients should be treated in the intensive care unit during the first 24–48 hours. Administration of low-dose insulin infusion as a part of DKA management requires intensive monitoring of blood glucose level, which is not applicable in a general ward. However, intensive care units are not available in every hospital in Indonesia. As the top national referral hospital in Indonesia, Cipto Mangunkusumo General Hospital has limited bed capacity in its intensive care unit. This may hinder the implementation of standard treatment for DKA patients, in particular admission to the intensive care unit. This is the reason why developing a model to predict mortality risk in DKA patients is very important.
Some studies have focused on factors associated with DKA mortality. However, not all of these factors were significant after further statistical analysis, and the significance of these factors was not always consistent when included in other studies. The screening systems available for rating the severity of DKA differ between countries and generally include only laboratory parameters. This may cause bias because laboratory parameters do not always correlate significantly with mortality.[5],[21]-[22] The preexisting prognostic score used by Efsthatiou et al.,[9] included variables such as pH <7.0, regular insulin dose >50 IU within the first 12 hours, serum glucose level >16.7 mmol/L after 12 hours, decreased level of consciousness, and fever within 24 hours. However, it can be difficult to implement this scoring system for estimating the mortality risk immediately after a patient’s arrival in the emergency room because some variables in this scoring system cannot be determined before the patient is treated.
The aim of our research was to develop a mortality prediction model for DKA patients in the first 72 hours after admission. The last step in the multivariate analysis identified four predictors of mortality in DKA patients: a history of DKA, comorbidities, level of consciousness, and lactate level. A history of DKA was significantly associated with 72-hour mortality in the multivariate analysis (p=0.020); HR 2.126 (95% CI 1.308–3.457). Some studies have found similar results. A study in Chicago classified patients according to the number of DKA episodes: first episode, 2–3 previous episodes, and ≥4 episodes. Mortality was higher in the group with the most previous episodes of DKA.23 Mills et al.[1] found that patients previously admitted to hospital with DKA had a 2.76-times higher risk of mortality within 21 months compared to those without a history of DKA. However, there is no well-defined pathophysiology that can explain the relationship between DKA recurrence and mortality. Recurrent episodes of DKA can be related to various causes, such as eating disorders, social problems, psychiatric disorders, and poor compliance with medication, as shown by a high HbA1c level.[13],[24]
Our study also found that patients with severe comorbidities (CCI score ≥5) had a mortality risk 2.4 times higher than those with mild to moderate comorbidities. This result is consistent with the results of a retrospective study by Ko et al.,[8] which found higher mortality in elderly subjects with other comorbidities, such as infection, liver failure, upper gastrointestinal bleeding, and cancer. Another retrospective cohort study by Barski et al.,[10] reported that death was not caused by the metabolic complication of DKA but instead resulted from the patients’ comorbid diseases, such as sepsis and multiple organ failure. Comorbidities are also included in the mortality prediction model by Efsthatiou et al.,[9] which found that patients with comorbidities had 16-times higher risk of death. Comorbidities might cause disruption to organ function, facilitate complications, and interfere with the implementation of the standard treatment protocol for DKA, which may later contribute to a higher mortality rate.
The level of consciousness is a clinical symptom that must be assessed in DKA patients. The Glasgow Coma Scale (GCS) is one of the best methods to assess the level of consciousness. Altered consciousness can be caused by several events including acidosis, increased osmolality, direct effects of ketone bodies, reduced blood supply to the brain, less uptake and use of glucose by brain cells, and other precipitating factors such as severe infection and stroke.[5],[25] Altered consciousness may also result from brain edema as a complication of fluid therapy in DKA.[26] Altered consciousness is one of the parameters used in determining the severity of DKA and is often associated with mortality. In our study, the level of consciousness was significantly associated with 72-hour mortality: HR 4.116 (CI 95% 2.048–8.270) (p<0.001) for a GCS score of 9–14 and HR 10.345 (CI 95% 4.860–22.019) (p<0.001) for a GCS score of 3–8. This suggests that a lower GCS score indicates a greater risk of death in DKA patients. Suwarto et al.,[5] also reported a similar finding. Venkatesh et al.,[12] found that the average GCS score was significantly higher in DKA survivors (GCS 14–16) compared with non-survivors (GCS 10) (p<0.0001).
Increased lactate level is used to assess the severity and predict mortality in various conditions. Lactate level is considered to be increased at >2 mmol/L and to be greatly increased at >4 mmol/L. Increasing lactate level may occur because of tissue hypoperfusion as a result of macro- and/or microcirculation dysfunction, mitochondrial dysfunction, hypermetabolic state, and disturbance of liver function.[27] In our study, 69.8% of patients with a lactate level of >4 mmol/L did not survive. A higher lactate level was significantly associated with mortality in the multivariate analysis: the HRs (95% CIs) were 3.117 (1.609–6.037) for a lactate level of 2–4 mmol/L (p=0.001) and 5.585 (2.966–10.519) for a lactate level of >4 mmol/L (p<0.001). Suwarto et al.,[5] also reported that lactate levels were higher in non-survivors (4.2 mmol/L) than in survivors (1.7 mmol/L). The role of lactate level in predicting mortality was also shown by Hendarto who reported that 80% of the patients who died within 24 hours of treatment had an initial blood lactate level of ≥2 mmol/L.[16]
The last step in the multivariate analysis in our study, which included a history of DKA, comorbidities, level of consciousness, and lactate level, was later applied to a scoring system in which the quality of each predictor corresponded to its association with mortality. The calculation was further divided by the smallest divisor value (value point) and ended with rounding the score into a natural number to ease the application of this model in everyday practice. This prediction model showed strong calibration and discrimination. Analysis of the contribution of the total score to the 72 hour mortality risk showed that for a total score of 0–2, the probability was 15.41%, for 3–4, the probability was 78.01%, and for 5–6, the probability was very high at 98.22%. These data show that a high total score indicates a high probability of death within 72 hours of admission to the emergency room.
Our mortality prediction model can be applied when identifying priority candidates for admission to the intensive care unit. This prediction model may provide a basis for clinicians deciding to admit DKA patients with a low mortality risk to the general ward. Clinicians from a primary healthcare or lower level hospital in Indonesia may handle DKA patients with low mortality risk without needing to refer them to a higher-level hospital. DKA patients with a higher risk of mortality could then be prioritized to receive more aggressive treatment.
Strengths of our study include a research sample over 6.5 years that comprised DKA patients with various types of DM. The predictor variables included both laboratory and clinical parameters, such as the level of consciousness, comorbidities, and age. This study also included multivariate analysis and a prediction model, and appears to be the first of its kind in Indonesia. The prediction model derived from this study had good internal validation for estimating 72-hour mortality risk in DKA patients.
Our study had some limitations. This was a retrospective cohort study with data obtained from medical and electronic health records in Cipto Mangunkusumo General Hospital. Some predictors such as the type of DM could not be confirmed by beta-cell function testing or the presence of antibody to insulin, which may have introduced bias into the study. A retrospective study also makes it difficult to evaluate nonclinical parameters, such as the referral system, which may have affected the medical service for each patient. The retrospective design of this study also led to difficulty in testing the predictive ability of the model. In addition, our study was completed in the top referral hospital in Jakarta, and the collected data may not therefore be representative of all DKA patients in all hospitals across the country. This mortality prediction model may only be applied in type 2 DM, since most of our subjects (90%) were type 2 DM patients. Finally, with regards to limitations of this study, further studies with prospective design are still needed to directly validate the performance of mortality prediction models in DKA patients.
The 72-hour mortality rate in DKA patients in Cipto Mangunkusumo General Hospital was 28.57%. Factors associated with mortality of DKA patients in the first 72 hours are a previous history of DKA, comorbidities, level of consciousness, and lactate level. Inclusion of these four predictors produced a prediction model for 72-hour mortality in DKA patients with strong performance.
Statement of AuthorshipAll authors certified fulfillment of ICMJE authorship criteria.
Author DisclosureThe authors declared no conflict of interest.
Funding SourceNone.
[1] Jervis A, Champion S, Figg G, Langley J, Adams GG. Prevalence of diabetic ketoacidosis rises and still no strict treatment adherence. Curr Diabetes Rev. 2013;9(1):54-61. PubMed
[2] Gosmanov AR, Gosmanova EO, Dillard-Cannon E. Management of adult diabetic ketoacidosis. Diabetes Metab Syndr Obes. 2014;7:255–64. PubMed PubMed Central
[3] Alourfi Z, Homsi H. Precipitating factors, outcomes, and recurrence of diabetic ketoacidosis at a university hospital in Damascus. Avicenna J Med. 2015;5(1):11–5. PubMed Pubmed Central
[4] Kitabchi AE, Umpierrez GE, Miles JM, Fisher JN. Hyperglycemic crises in adult patients with diabetes. Diabetes Care. 2009;32(7):1335–43. PubMed Central
[5] Suwarto S, Sutrisna B, Waspadji S, Pohan H. Predictors of five-day mortality in diabetic ketoacidosis patients: A prospective cohort study. Acta Med Indones. 2014;46(1):18–23. PubMed
[6] MacIsaac RJ, Lee LY, McNeil KJ, Tsalamandris C, Jerums G. Influence of age on the presentation and outcome of acidotic and hyperosmolar diabetic emergencies. Intern Med J. 2002;32(8):379-85. PubMed
[7] Henriksen OM, Røder ME, Prahl J, Svendsen OL. Diabetic ketoacidosis in Denmark incidence and mortality estimated from public health registries. Diabetes Res Clin Pract. 2007;76(1):51–6. PubMed CrossRef
[8] Ko SH, Lee WY, Lee JH, et al. Clinical characteristics of diabetic ketoacidosis in Korea over the past two decades. Diabet Med. 2005;22(4):466–9. PubMed CrossRef
[9] Efstathiou SP, Tsiakou AG, Tsioulos DI, et al. A mortality prediction model in diabetic ketoacidosis. Clin Endocrinol (Oxf). 2002;57(5):595–601. PubMed
[10] Barski L, Nevzorov R, Rabaev E, et al. Diabetic ketoacidosis: Clinical characteristics, precipitating factors and outcomes of care. Isr Med Assoc J. 2012;14(5):299-303. PubMed
[11] Mills LS, Stamper JE. Adult diabetic ketoacidosis: Diagnosis, management and the importance of prevention. J Diabetes Nurs. 2014;18(1):8-12. https://www.diabetesonthenet.com/uploads/resources/dotn/_master/3613/files/pdf/jdn18-1-8-12.pdf
[12] Venkatesh B, Pilcher D, Prins J, Bellomo R, Morgan TJ, Bailey M. Incidence and outcome of adults with diabetic ketoacidosis admitted to ICUs in Australia and New Zealand. Critical Care. 2015;19:451. PubMed PubMed Central
[13] Gibb FW, Teoh WL, Graham J, Lockman KA. Risk of death following admission to a UK hospital with diabetic ketoacidosis. Diabetologia. 2016;59(10):2082–7. PubMed PubMed Central CrossRef
[14] Agarwal A, Yadav A, Gutch M, et al. Prognostic factors in patients hospitalized with diabetic ketoacidosis. Endocrinol Metab (Seoul). 2016;31(3):424-32. PubMed PubMed Central CrossRef
[15] Kakusa M, Kamanga B, Ngalamika O, Nyirenda S. Comatose and noncomatose adult diabetic ketoacidosis patients at the university teaching hospital, Zambia: Clinical profiles, risk factors and mortality outcomes. Indian J Endocrinol Metab. 2016;20(2):199-205. PubMed PubMed Central CrossRef
[16] Hendarto H. Gambaran kadar laktat darah pada penderita ketoasidosis diabetik yang meninggal pada 24 jam pertama di Instalasi Gawat Darurat RSUPN-CM antara bulan Maret-Agustus 2002. Thesis. Universitas Indonesia: 2003.
[17] Huang YQ, Gou R, Diao YS, et al. Charlson comorbidity index helps predict the risk of mortality for patients with type 2 diabetic nephropathy. J Zhejiang Univ Sci B 2014;15(1):58-66. PubMed PubMed Central CrossRef
[18] Umpierrez GE, Murphy MB, Kitabchi AE. Diabetic ketoacidosis and hyperglycemic hyperosmolar syndrome. Diabetes Spectr. 2002;15(1):28-36. CrossRef
[19] Chiasson JL, Aris-Jilwan N, Bélanger R, et al. Diagnosis and treatment of diabetic ketoacidosis and the hyperglycemic hyperosmolar state. CMAJ. 2003;168(7):859–66. PubMed PubMed Central
[20] Waspadji S. Penatalaksanaan Kedaruratan di Bidang Ilmu Penyakit Dalam. PIP Bagian Ilmu Penyakit Dalam FKUI RSUPNCM, 2000.
[21] Snorgaard O, Eskildsen PC, Vadstrup S, Nerup J. Diabetic ketoacidosis in Denmark: Epidemiology, incidence rates, precipitating factors and mortality rates. J Intern Med.1989;226(4):223–8. CrossRef
[22] Ellemann K, Soerensen JN, Pedersen L, Edsberg B, Andersen OO. Epidemiology and treatment of diabetic ketoacidosis in a community population. Diabetes Care. 1984;7(6):528–32. CrossRef
[23] Mays JA, Jackson KL, Derby TA, et al. An evaluation of recurrent diabetic ketoacidosis, fragmentation of care, and mortality across Chicago, Illinois. Diabetes Care. 2016;39(10):1671-6. PubMed CrossRef
[24] Cooper H, Tekiteki A, Khanolkar M, Braatvedt G. Risk factors for recurrent admissions with diabetic ketoacidosis: A case-control observational study. Diabet Med. 2016;33(4):523–8. PubMed CrossRef
[25] Pishdad G, Ghavanini AA. Factors contributing to alterations in the level of consciousness in patients with diabetic ketoacidosis: Analysis of 189 cases. Med J Islam Repub Iran. 1999;13(2):699-702.
[26] Nyenwe EA, Razavi LN, Kitabchi AE, Khan AN, Wan JY. Acidosis: The prime determinant of depressed sensorium in diabetic ketoacidosis. Diabetes Care. 2010; 33(8):1837–9. PubMed PubMed Central CrossRef
[27] Andersen LW, Mackenhauer J, Roberts JC, Berg KM, Cocchi MN, Donnino MW. Etiology and therapeutic approach to elevated lactate. Mayo Clin Proc. 2013;88(10):1127–40. PubMed PubMed Central NIHMSID: NIHMS564727 CrossRef
Authors are required to accomplish, sign and submit scanned copies of the JAFES Author Form consisting of: (1) Authorship Certification, that all the requirements for authorship have been met by each author, and that the final version of the manuscript has been read and approved by all authors; (2) the Author Declaration, that the article represents original material that is not being considered for publication or has not been published or accepted for publication elsewhere; (3) the Statement of Copyright Transfer [accepted manuscripts become the permanent property of the JAFES and are licensed with an Attribution-Share Alike-Non-Commercial Creative Commons License. Articles may be shared and adapted for non-commercial purposes as long as they are properly cited]; and the ICMJE form for Disclosure of Potential Conflicts of Interest. For original articles, authors are required to submit a scanned copy of the Ethics Review Approval of their research as well as registration in trial registries as appropriate. For manuscripts reporting data from studies involving animals, authors are required to submit a scanned copy of the Institutional Animal Care and Use Committee approval. For Case Reports or Series, and Images in Endocrinology, consent forms, are required for the publication of information about patients; otherwise, appropriate ethical clearance has been obtained from the institutional review board. Articles and any other material published in the JAFES represent the work of the author(s) and should not be construed to reflect the opinions of the Editors or the Publisher.