Association of Dialysis Malnutrition Score with Hypoglycemia and Quality of Life Among Patients with Diabetes on Maintenance Hemodialysis
Melissa Claire Uy,* Rebecca Lim-Alba,* Eric Chua**
Melissa Claire K. Uy, MD
Section of Endocrinology, Diabetes and Metabolism
Department of Medicine, Chinese General Hospital and Medical Center
286 Blumentritt Rd, Sta. Cruz, Manila, Philippines 1014
Tel. No.: +632-711-4141 local 536
E-mail: mclaireuy@gmail.com
ORCID: https://orcid.org/0000-0001-8622-5531
e-ISSN 2308-118x
Printed in the Philippines
Copyright © 2018 by the JAFES
Received April 19, 2018. Accepted July 6, 2018.
Published Online First: September 9, 2018.
Objective. To determine the association between Dialysis Malnutrition Score (DMS), hypoglycemia and quality of life among patients with Diabetes on Maintenance Hemodialysis (MHD).
Methodology. Ninety-two diabetic patients on maintenance hemodialysis were assessed using a standardized data collection tool, Dialysis Malnutrition Score, WHOQoL-BREF questionnaire, anthropometric measurements and hourly blood sugar monitoring during the dialysis session. Association among DMS, hypoglycemia and quality of life were assessed along with other associated variables.
Results. Based on the DMS, 62% of patients were malnourished. Those with malnutrition were significantly older (p=0.0006) and female (p=0.013). Only 6.5% of the participants developed hypoglycemia during dialysis. Those with poor nourishment in the DMS showed a significant trend of decrease in the quality of life (physical (p<0.001), psychological (p<0.001) and social (p=0.004) and is associated with the occurrence of hypoglycemia (p<0.001).
Conclusion. Malnutrition is prevalent in diabetic patients on MHD using DMS. A higher DMS score is highly correlated with increased risk of hypoglycemia and decreased quality of life hence detection of malnutrition is important to prevent further nutritional depletion, hypoglycemia and poor patient outcomes by implementing preventive measures such as nutritional counselling and psychosocial interventions.
Keywords: malnutrition, Dialysis Malnutrition Score, hypoglycemia, quality of life, dialysis
According to the International Diabetes Federation, in 2017, 425 million adults are currently estimated to have diabetes and the Philippines is one of the world’s emerging diabetes hotspots with 3.7 million Filipinos diagnosed with the disease.[1] The increasing prevalence of diabetes has also led to an increase in the number of complications such as diabetic kidney disease (DKD). DKD, which occurs in 20-40% of all diabetics,[2] is the single strongest predictor of mortality in patients with diabetes and remains to be the most common reason for progressing to end stage renal disease (ESRD) requiring maintenance hemodialysis (MHD).[3],[4] DKD predisposes patients to protein energy wasting (PEW), with a 40-70% prevalence among patients on MHD.[5],[6],[7] PEW is associated with deterioration of disease condition, impaired wound healing, predisposition to hypoglycemia, depression, increased morbidity, mortality, hospitalization rate and susceptibility to infection which results in poor quality of life.[8],[9],[10],[11] Routine screening of PEW in dialysis patients is seldom done because of difficulty of an accurate determination of the nutritional status which requires procedures such as anthropometric, body composition and biochemical measurements; and functional, dietary and subjective assessments which are time consuming, not cost effective and inconvenient to most dialysis centers.[12], [13]
There are different tools used to detect PEW in patients with MHD and these were proven to be correlated with dietary intake, anthropometric measurements and laboratory assessments related to nutrition.[7],[10],[13], [14], [15] Previously, the Subjective Global Assessment (SGA) was the most commonly used method but is only semi-quantitative which has restricted reliability and precision.17 Subsequently, a fully quantitative nutritional scoring system, the Modified Subjective Global Assessment or Dialysis Malnutrition Score (DMS) and Malnutrition Inflammation Score (MIS), were developed which incorporated the advantages of SGA and extended its reliability and precision.[16]
DMS consists of 7 variables such as weight change, dietary intake, gastrointestinal symptoms, functional capacity, co-morbidity, decreased fat stores and signs of muscle wasting. MIS, on the other hand, includes the 7 components of the DMS plus 3 new components: BMI, serum albumin and TIBC. Both the DMS and MIS correlated significantly in MHD patients and are valid tools to be used for nutrition screening, it also has advantage that it can detect small changes in nutrition status overtime which can guide the physician for the assessment of nutrition intervention.[16] DMS and MIS has a sensitivity of 94% and 87% and specificity of 88% and 96%, respectively in comparison with SGA.[17] With these tools, detection of malnutrition can be easily done within minutes. DMS is a more sensitive, practical and simpler tool to detect malnutrition in routine hospital assessments.[14]
There are very limited local studies regarding the nutritional status of dialysis patients and the use of such tools to detect malnutrition reliably and conveniently. DMS can be used in all dialysis centers; it can anticipate early nutritional depletion, help the physician prevent any health deterioration, morbidity and mortality by implementing preventive measures such as nutritional counselling and psychosocial interventions which can reduce the risk of complications and can be valuable towards improving quality of life and patients’ outcomes.
Objectives
This study aims to determine the association between Dialysis Malnutrition Score (DMS), hypoglycemia and quality of life among patients with Diabetes on Maintenance Hemodialysis (MHD) in Chinese General Hospital and Medical Center. It also aimed to determine the following: the prevalence of malnutrition among patients with Diabetes on MHD using DMS, the prevalence of hypoglycemia among patients with DKD on MHD, the quality of life of patients with DKD on MHD using WHOQoL-BREF questionnaire, the correlation between DMS and WHOQoL-BREF in diabetic patients on MHD and the association between the occurrence of hypoglycemia and DMS in diabetic patients on MHD.
METHODOLOGYThis is a cross-sectional study conducted between August to November 2017 at the Hemodialysis Unit of Chinese General Hospital and Medical Center, Manila, Philippines. All adult patients, at least 18 years of age with Diabetes Mellitus on maintenance hemodialysis for at least 3 months were included. The exclusion criteria were as follows: kidney transplant patients, acute infection or sepsis, multi-organ failure, coma, hospitalization in the last 3 months, ongoing oral or parenteral nutritional supplementation, use of steroidal, anti-inflammatory or immunosuppressive agents, receiving protein supplementation including amino acids or any nutritional supplements except for folic acid within 3 months prior to enrollment, history of psychological disorder such as schizophrenia and patients participating in other studies involving nutrition.
Data collection tools and methods
Patient's data
Written informed consent was obtained from a total of 92 participants. A standardized data collection tool was prepared for each subject and data was collected prospectively through interview, review of medical records and laboratory data from the dialysis charts. The questionnaire included the patient’s age, gender, occupation, civil status, educational background, financial status, hemodialysis schedule, hemodialysis duration, diabetes duration, creatinine, serum albumin, comorbid conditions and medications (Appendix 1).

Appendix 1. Data Collection Form
Dialysis Malnutrition Score (DMS)
A validated Modified Subjective Global Assessment or Dialysis Malnutrition Score (DMS) (Boado J et al., Nutritional Assessment of patients on maintenance hemodialysis using Dialysis Malnutrition Score) consists of 7 features: weight change, dietary intake, GI symptoms, functional capacity, co-morbidity, subcutaneous fat and signs of muscle wasting (Appendix 2). Patients were interviewed and charts reviewed to gather the pertinent medical history. For weight change, the overall change in the post dialysis dry weight was obtained. The lowest score of one was given if there was no weight change or if patient had gained weight. Score of two was given for minor weight loss (<5%), score of three for weight loss of >10%, score of four for weight loss of 10-15% and score of five for any weight loss over 15% in the last 6 months. Dietary intake was scored one if it was considered as a regular solid intake with no recent change in the amount or quality of the meals, two for sub-optimal solid diet, three for full liquid diet or any moderate overall decrease, four for hypocaloric liquid and five for starvation. Gastrointestinal (GI) symptoms were scored one if there was no symptom, two for nausea, three for vomiting or any moderate GI symptoms, four for diarrhea and five for severe anorexia. Functional capacity was scored one for normal functional capacity and/or any considerable improvement in the level of previous functional impairment, two for any mild to moderate difficulty with ambulation, three for difficulty with normal activity, four for difficulty with light activity and five for bed/chair-ridden state. The co-morbidity was scored one if there was no medical problems and if the patient has been on MHD for less than one year; two if there was mild co-morbidity or if the patient has been on MHD for one to two years; three if there was moderate co-morbidity or if the patient had been dialyzed for two to four years, or if the patient was >75 years of age; four if there was severe co-morbidity or if the patient had been dialyzed for over four years; and five if there were very severe, multiple co-morbidities. Subcutaneous fat was scored by assessing subcutaneous fat deposition in four body areas: below the eyes, triceps, biceps and chest. Signs of muscle wasting were obtained by examining the temple, clavicle, scapula, ribs and quadriceps. Each component was assigned a score from 1 (normal), 2 to 4 (moderate malnutrition) and 5 (severe malnutrition). A lower score (7-10) denotes tendency towards a normal nutritional status while a higher score (>10) is considered to be an indicator of the severity of malnutrition.
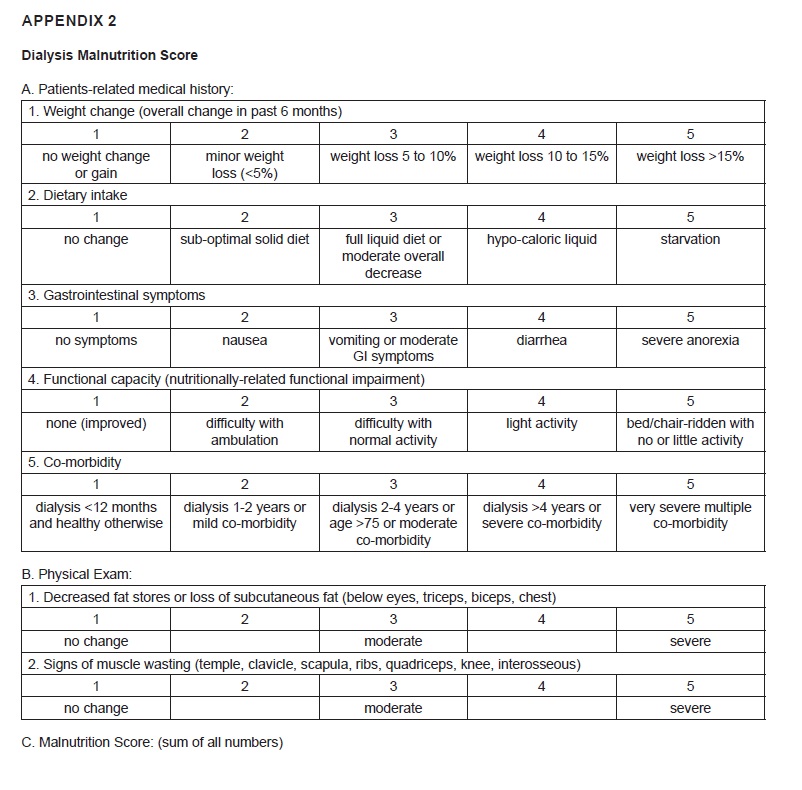
Appendix 2. Dialysis Malnutrition Score
Anthropometric measurement
Body dry weight, height and skin-fold measurements were performed immediately after termination of dialysis session. Triceps skin–fold (TSF) in millimeters was measured using skin–fold caliper and Mid-arm circumference (MAC) in centimeters was measured using a tape measure. Body mass index was calculated using the formula kg/m2. All the above measurements were performed two times on the non-access arm of each dialysis patient and the average result of the two measurements were registered as the final result.
Blood glucose measurement
During one session of the participant’s dialysis, the serum glucose levels were measured immediately before starting hemodialysis and hourly until the end of the session using One Touch Select glucose meter and glucose meter strips manufactured by Johnson and Johnson. Values less than 70 mg/dL were considered as hypoglycemia with or without symptoms.
Quality of Life
All subjects were provided with a validated WHOQOL-BREF questionnaire in Filipino (Dela Vega, S. Improving the quality of life of Filipinos) for the assessment of quality of life for patients on Hemodialysis. WHOQOL-BREF Questionnaire was developed by the WHOQOL Group with fifteen internal field centers, simultaneously in an attempt to develop a quality of life assessment. It has 4 major domains: physical health, psychological, social relationships and environment with two individually scored items about individual’s overall perception of quality of life and health. The four domain scores are scaled in a positive direction with higher scores indicating a higher quality of life. The 4 domains are then scored, labeled and transformed to a 0-100 scale using the transformation scale score (Appendix 3).
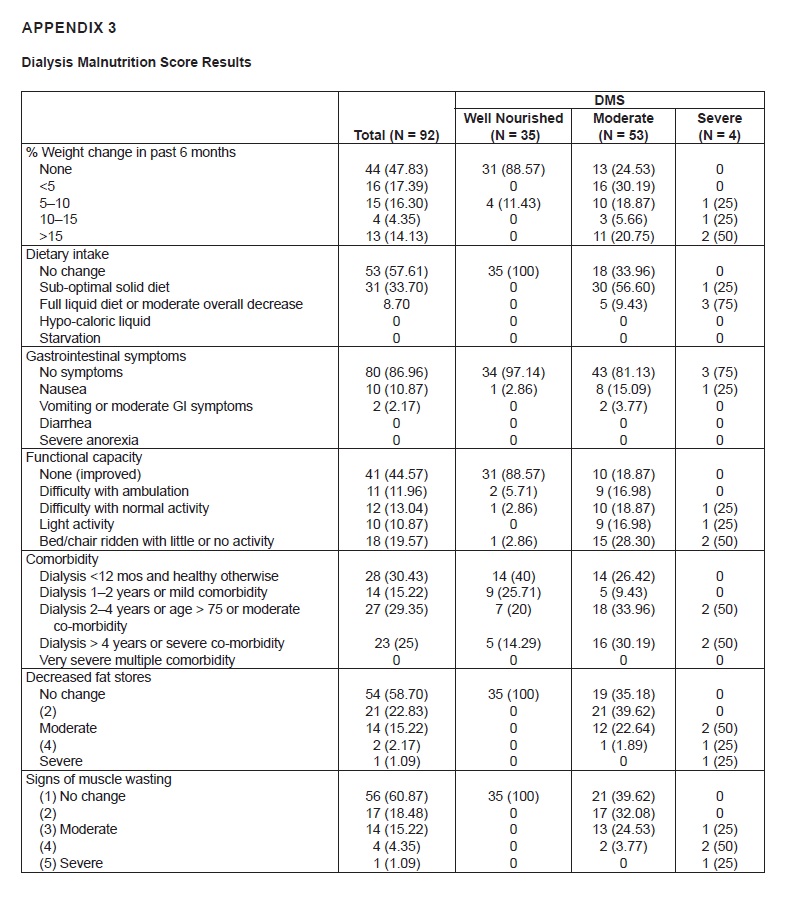
Appendix 3. Dialysis Malnutrition Score Results
Statistical Analysis
We computed a sample size requirement of 91 subjects, based on 90% power and 5% level of significance to detect a correlation coefficient of 0.334 from the reference article by Sohrabi Z.[11] Descriptive statistics were used to summarize the clinical characteristics of the patients. Frequency and proportion were used for nominal variables, median and range for ordinal variables, and mean and SD for interval/ratio variables. Spearman’s rank correlation was used to determine the correlation between DMS and QoL scores. Logistic regression was initially planned to determine predictors of malnutrition in diabetic ESRD patients on maintenance hemodialysis, but it was impractical due to a very low number of patients with hypoglycemia in our study. All valid data were included in the analysis. Missing variables were neither replaced nor estimated. The null hypothesis was rejected at 0.05 α-level of significance. STATA 15.0 was used for data analysis.
Ethical considerations
This study was conducted in accordance to the ethical principles based on the Declaration of Helsinki and the National Guidelines for Biomedical Research of the National Ethics Committee (NEC) of the Philippines. This study was approved by the Research and Ethics Review Board (RERB). All patients provided written informed consent.
Among the 180 patients on maintenance hemodialysis, there were 101 patients with diabetes. Five were excluded due to hospitalization because of acute infection, 4 were on enteral feeding, hence, a total of 92 patients were included in the analysis. Of the 92 patients, there were 35 (38%) patients who were classified as well-nourished by DMS scoring, 53 (57.6%) who were moderately malnourished, and four (4.35%) severely malnourished. Overall, they had a median age of 69 years, median duration of diabetes of 10 years, and 48.91% were female, less than 10% were working, and the majority were able to finish college (49%).
Their baseline socio-demographics and anthropometrics are presented in Table 1.
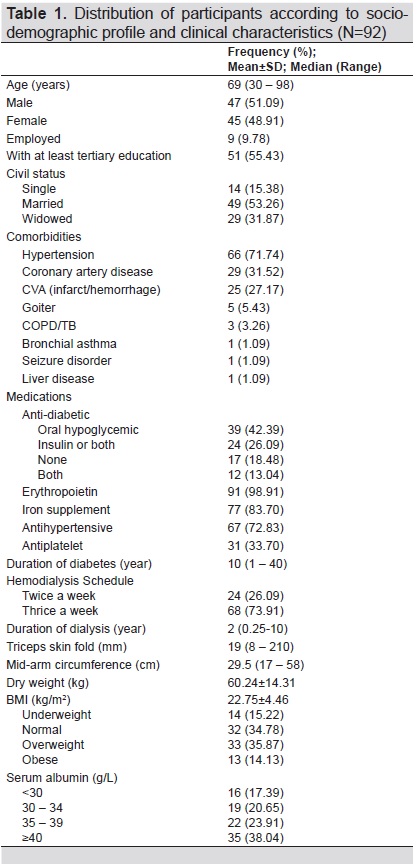
Table 1. Distribution of participants according to socio-demographic profile and clinical characteristics (N=92)
A greater proportion among those with malnutrition were older (71 years versus 63 years, p<0.001), were female (57.89% versus 34.29%, p=0.028), and were widowed (42.11% versus 14.29%, p=0.014). We had insufficient evidence to demonstrate a difference between groups in terms of employment, education, and duration of dialysis (Table 2).
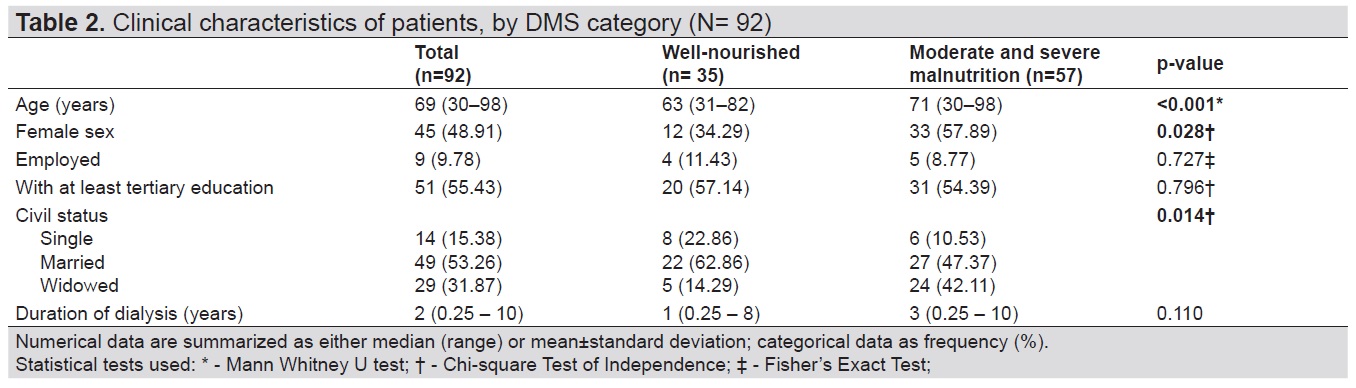
Table 2. Clinical characteristics of patients, by DMS category (N= 92)
Of 92 patients, there were six patients (6.52%) who had hypoglycemia, four of whom were moderately malnourished and two who were severely malnourished. WHOQoL-BREF scores are presented on Table 3. WHOQoL-BREF scores range from zero to 100, with higher scores indicating better quality of life. The quality of life scores were relatively low, scoring below 60 points overall and across domains. The well-nourished group had significantly higher scores in physical, psychological, and social relationships domains.
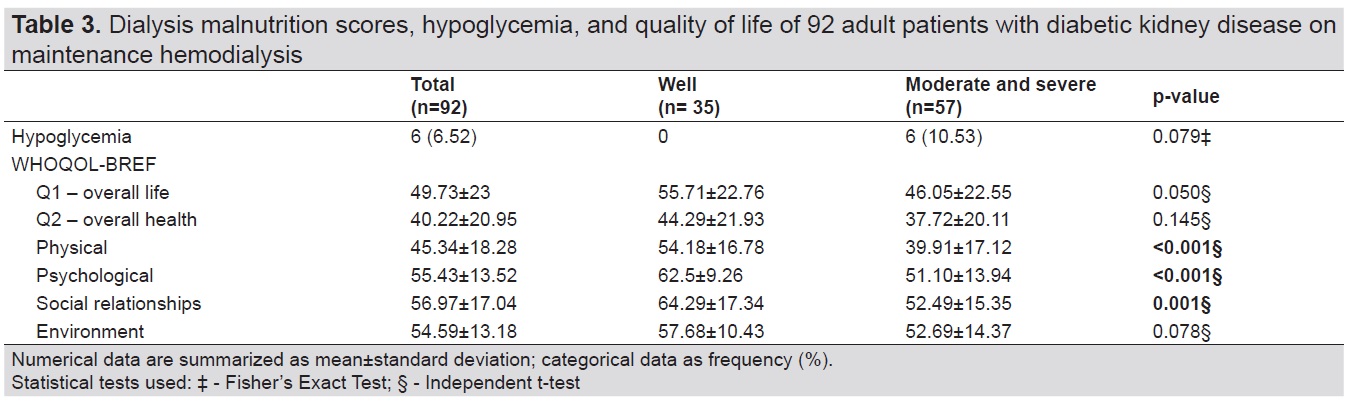
Table 3. Dialysis malnutrition scores, hypoglycemia, and quality of life of 92 adult patients with diabetic kidney disease on maintenance hemodialysis
We observed statistically significant and negative weak to moderate correlations between DMS and overall life, and on the domains of physical, psychological, and social relationships (Table 4).
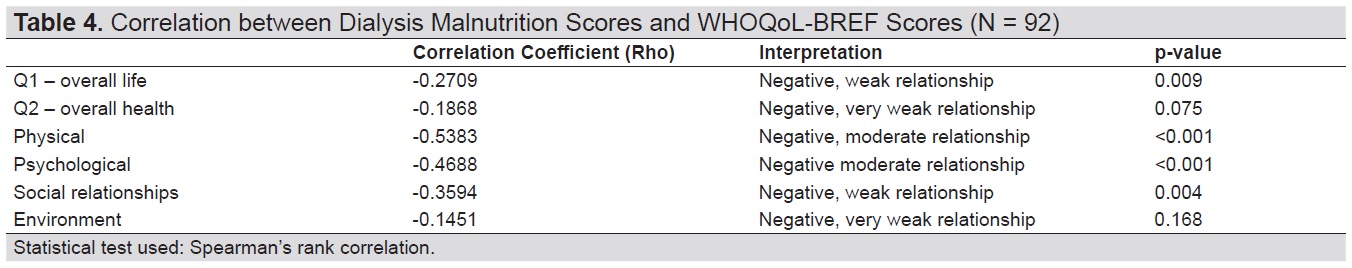
Table 4. Correlation between Dialysis Malnutrition Scores and WHOQoL-BREF Scores (N = 92)
DMS stratifies patients into well nourished, moderately malnourished and severely malnourished which has an impact on patients’ outcome when not detected and properly addressed. In this study, based on the DMS, 58% had moderate malnutrition and 4% were severely malnourished. Using the DMS, in the study of Afshar et al., which included 54 patients on MHD in Iran, 35% had moderate malnutrition and 6% had severe malnutrition[10] while that of Soodeh et al., had 67% malnutrition rate among the 112 chronic hemodialysis Iranian patients.[18]
It appears that the nutritional status of our patients are almost similar with other countries. There was only one study done in the Philippines using DMS for hemodialysis patients done by Boado et al. It included 33 patients on MHD, of which 81% had malnutrition.[12] It had higher rate of malnutrition compared to our study probably because a large proportion of the population (88%) were on twice a week dialysis in contrast to our population wherein majority (74%) were on thrice a week hemodialysis schedule.[12] In the study of Divina et al., the lesser frequency of dialysis showed significant association with the development and severity of malnutrition due to inadequacy of dialysis.[7]
An older age and female sex predispose patients to malnutrition such as in the study of Miguel et al.21 In the study by Kalantar-Zadeh et al., women also had a stronger tendency to malnutrition but was not significant.[6] Some studies have found that age has an adverse effect on the incidence of malnutrition which can be due to underlying psychological disorders such as depression and economic or physical disability in the preparation and consumption of food.[9] Many changes associated with the process of aging can promote malnutrition and is frequently associated with decreases in taste acuity and smell, deterioration in dental health and decrease in physical activity which may affect nutrient intake.[20] In the study of Boado et al., and Sohrabi et al., dialysis duration was not associated with malnutrition which is the same in this study.[11]
In our study, overall life and health status appeared to be lower with poorer state of nutrition. The trends of the physical domain scores (pain and discomfort, energy, sleep), psychological health (positive feelings, memory and concentration, self esteem, bodily image and appearance, negative feelings) and social relationships (personal and social support, sexual activity) of the malnourished group were notably lower as compared to the well-nourished group. In the study of Rambod et al., negative correlations were found between nutritional status and quality of life aspects,[21] Bilgic et al., also found a significant association between MIS and poor quality of life[22] and Spiegel et al., showed that nutritional biomarkers were correlated with quality of life[23] which are all consistent with this study. Quality of life is a predictor of survival in HD patients[24] hence correlation between nutritional status using the DMS and quality of life focuses on the effects of malnutrition status on patients’ survival. The decrease in quality of life is an important determinant of hospitalization and death in patients on MHD. Our study is also similar to the study of Sathvik et al., which used the WHOQOL-BREF in 75 hemodialysis patients from India, the evidence did not support a significant difference in mean environment (safety and security, home and physical environment) score across groups because most of the patients revealed that they have enough time for recreational activities with their families and they have a decent home or physical environment. They were also satisfied with their access to health services in the hospital.[25]
Screening for malnutrition using DMS is of utmost importance not only because it is the first step to correct malnutrition but also because it can prompt a reduction of unnecessary anti-hyperglycemic therapy preventing hypoglycemic episodes because hypoglycemia is associated with significant morbidities leading to both physical and cognitive dysfunction and further deterioration of patients’ general health.[26],[27],[28] In our study, only 6 participants or 6.5% had episode of hypoglycemia without symptoms during dialysis. Four of those who developed hypoglycemia were on sulfonylurea while 2 of the participants were on insulin. Two out of the 6 participants who developed hypoglycemia also had episodes of hypoglycemia at home probably due to a delay in the metabolism and excretion of insulin and oral hypoglycemic agents. This is less than the 15.2% in the study of Cho et al., which included 1685 Asian patients with or without diabetes on hemodialysis and peritoneal dialysis for at least 1 month.[27] This is probably due to the difference in population studied. In Cho et al.’s study, 74% of the patients who had hypoglycemia were diabetics and they also reported that 15.6% of patients with hypoglycemia had clinical malnutrition.[27] Patients with DMS detected malnutrition often have poor appetite, decreased hepatic glycogen stores, reduced availability of gluconeogenic, insulin resistance and glucose intolerance which can lead to decreased weight and hypoglycemia hence evaluation of nutritional status using DMS with optimal dose of dialysis is important to prevent PEW and subsequent hypoglycemia.[28],[29],[30],[31]
Malnutrition is prevalent in diabetic patients on MHD using DMS which calls for more attention to early identification and management. A higher DMS score is highly correlated with increased risk of hypoglycemia and decreased quality of life hence detection of malnutrition is important to prevent further nutritional depletion, hypoglycemia and poor patient outcomes by implementing preventive measures such as nutritional counselling and psychosocial interventions.
LimitationsThere was limited sample size and the adequacy of dialysis (Kt/V) was not determined because of lack of available data and funding. The participants also have different timing of dialysis or shifts which may affect the detection of hypoglycemia. The causality of the association between DMS, hypoglycemia and quality of life cannot be proven, which is an inherent nature of cross-sectional studies.
RecommendationsFurther studies with larger sample size, same dialysis shifts and equal number of participants in each classification of DMS are suggested to decrease bias. More longitudinal studies are needed to assess the association of DMS with hypoglycemia, quality of life and related risk factors.
AcknowledgmentThe authors would like to thank their dietitian, Mr. Emman Mistades for helping them with the dietary data and Dr. Venus Cloma-Rosales for helping them with the statistics and in the interpretation of the WHOQOL-BREF questionnaire and calculating the scores.
Statement of AuthorshipAll authors certified fulfillment of ICMJE authorship criteria.
Author DisclosureThe authors declared no conflict of interest.
Funding SourceNone.
[1] International Diabetes Federation, Diabetes Atlas, 8th edition, 2017.
[2] Gheith O, Farouk N, Nampoory N, et al. Diabetic kidney disease: Worldwide difference of prevalence and risk factors. J Nephropharmacol. 2016;5(1):49-56. PubMed PubMed Central
[3] Toth-Manikowski S, Atta MG. Diabetic kidney disease: Pathophysiology and therapeutic targets. J Diabetes Res V. 2015;2015:Article ID 697010. CrossRef
[4] Ghaderian SB, Hayati F, Shayanpour S, Beladi Mousavi SS. Diabetes and end-stage renal disease; A review article on new concepts. J Renal Inj Prev. 2015;4(2):28-33. PubMed PubMed Central CrossRef
[5] Ikizler T. Optimal nutrition in hemodialysis patients. Adv Chronic Kidney Dis. 2013; 20(2):181-9. PubMed PubMed Central CrossRef
[6] Kalantar-Zadeh K, Kleiner M, Dunne E, Lee GH, Luft FC. A modified quantitative subjective global assessment of nutrition for dialysis patients. Nephrol Dial Transplant 1999;14(7):1732-8. PubMed
[7] Divina RJ, Felicida FS, Chan RE, Llido LO. Nutritional status of hemodialysis patients in the Philippines: A cross-sectional survey in four out-patient dialysis centers. PhilsPEN. 2010;82-9.
[8] Czira M, Lindner AV, Szeifert L, et al. Association between the malnutrition-inflammation score and depressive symptoms in kidney transplanted patients. Gen Hosp Psychiatry. 2011;33(2):157-65. PubMed
[9] Ebrahimzadehkor B, Dorri A, Yapan-Gharavi A. Malnutrition-inflammation score in hemodialysis patients. Zahedan J Res Med Sci. 2014;16(8):25-8.
[10] Afshar R, Sanavi S, Izadi-Khah A. Assessment of nutritional status in patients undergoing maintenance hemodialysis: A single-center study from Iran. Saudi J Kidney Dis Transpl.2007;18(3):397-404. PubMed
[11] Sohrabi Z, Eftekhari MH, Eskandari MH, Rezaeianzadeh A, Sagheb MM . Malnutrition-inflammation score and quality of life in hemodialysis patients: Is there any correlation? Nephrourol Mon. 2015;7(3):e27445. PubMed PubMed Central CrossRef
[12] Boado JRV, Redondo DC, Flauta-Orio J, et al. Nutrition assessment of patients on maintenance hemodialysis using dialysis malnutrition score (DMS). PhilSPEN 2014;74-88.
[13] Yamada K, Furuya R, Takita T, et al. Simplified nutritional screening tools for patients on maintenance hemodialysis. Am J Clin Nutr. 2008;87(1):106-13. PubMec CrossRef
[14] Harvinder GS, Swee WC, Karupaiah T, et al. Dialysis malnutrition and malnutrition inflammation scores: Screening tool for prediction of dialysis related protein-energy wasting in Malaysia. Asia Pac J Clin Nutr 2016;25(1):26-33. PubMed
[15] As’habi A, Tabibi H, Nozary-Heshmati B, Mahdavi-Mazdeh M, Hedayati M. Comparison of various scoring methods for the diagnosis of protein-energy wasting in hemodialysis patients. Int Urol Nephrol. 2014;46:999-1004.
[16] Lim S, Lin XH, Daniels L. Seven-point subjective global assessment is more time sensitive than conventional subjective global assessment in detecting nutrition changes. JPEN J Parenter Enteral Nutr. 2016;40(7):966-72. PubMed CrossRef
[17] Campbell KL, Ash S, Bauer J, Bauer J, Davies PSW. Critical review of nutrition assessment tools to measure malnutrition in chronic kidney disease. Nutrition and Dietetics. 2007;64(1):22-30. CrossRef
[18] Jahromi SR, Hosseini S, Razeghi E, Meysamie Ap, Sadrzadeh H. Malnutrition predicting factors in hemodialysis patients. Saudi J Kidney Dis Transpl. 2010;21(5):846-51. CrossRef
[19] Miguel LS, Julia ÁH, Mercè P, et al. Prevalence of hospital malnutrition in patients with diabetes mellitus: A sub-analysis of the PREDyCES study. SM J Public Health Epidemiol. 2015;1(4):1018.
[20] Hickson M. Malnutrition and ageing. Postgrad Med J. 2006;82(963):2-8. CrossRef PubMed Central CrossRef
[21] Rambod M, Bross R, Zitterkoph J, et al. Association of malnutrition-inflammation score with quality of life and mortality in hemodialysis patients: A 5-year prospective cohort study. Am J Kidney Dis. 2009; 53(2):298-309. PubMed PubMed Central NIHMSID: NIHMS322402.
[22] Bilgic A, Akgul A, Sezer S, Arat et al. Nutritional status and depression, sleep disorder and quality of life in hemodialysis patients. J Ren Nutr 2007;17(6):381-8. PubMed CrossRef
[23] Spiegel BMR, Melmed G, Robbins S, Esrailian E. Biomarkers and health-related quality of life in end-stage renal disease: A systematic review. Clin J Am Soc Neohrol. 2008; 3(6):1759-68. PubMed PubMed Central
[24] Yusop NBM, Mun CY, Shariff ZM, Huat CH. Factors associated with quality of life among hemodialysis patients in Malaysia. PLoS ONE. 2013;8(12):e84152. CrossRef
[25] Sathvik BS, Parthasarathi G, Narahari MG, Gurudev KC. An assessment of the quality of life in hemodialysis patients using the WHOQOL-BREF questionnaire. Indian J Nephrol. 2008;18(4):141-9. PubMed PubMed Central CrossRef
[26] Chu YW, Lin HM, Wang JJ, Weng SF, Lin CC, Chien CC. Epidemiology and outcomes of hypoglycemia in patients with advanced diabetic kidney disease on dialysis: A national cohort study. PLoS One. 2017;12(3):e0174601. PubMed PubMed Central CrossRef
[27] Cho A, Noh JW, Kim JK, et al. Prevalence and prognosis of hypoglycaemia in patients receiving maintenance dialysis. CrossRef
[28] Abdelhafiz A, Rodriguez-Mañas L, Morley JE, Sinclair A. hypoglycemia in older people- a less well recognized risk factor for frailty. Aging Dis. 2015;6(2):156-67. PubMed PubMed Central CrossRef
[29] Jung HS, Kim HI, Kim MJ, et al. Analysis of hemodialysis-associated hypoglycemia in patients with type 2 diabetes using a continuous glucose monitoring system. Diabetes Tech Ther. 2010;12(10):801-7. PubMed CrossRef
[30] Abe M, Kalantar-Zadeh K. Hemodialysis-induced hypoglycemia and glycemic disarrays. Nat Rev Nephrol. 2015;11(5):302-12. PubMed PubMed Central CrossRef
[31] Sun CY, Lee CC, Wu MS. Hypoglycemia in diabetic patients undergoing chronic hemodialysis. Ther Apher Dial. 2009;13(2):95-102. PubMed CrossRef
Authors are required to accomplish, sign and submit scanned copies of the JAFES Author Form consisting of: (1) Authorship Certification, that all the requirements for authorship have been met by each author, and that the final version of the manuscript has been read and approved by all authors; (2) the Author Declaration, that the article represents original material that is not being considered for publication or has not been published or accepted for publication elsewhere; (3) the Statement of Copyright Transfer [accepted manuscripts become the permanent property of the JAFES and are licensed with an Attribution-Share Alike-Non-Commercial Creative Commons License. Articles may be shared and adapted for non-commercial purposes as long as they are properly cited]; and the ICMJE form for Disclosure of Potential Conflicts of Interest. For original articles, authors are required to submit a scanned copy of the Ethics Review Approval of their research as well as registration in trial registries as appropriate. For manuscripts reporting data from studies involving animals, authors are required to submit a scanned copy of the Institutional Animal Care and Use Committee approval. For Case Reports or Series, and Images in Endocrinology, consent forms, are required for the publication of information about patients; otherwise, appropriate ethical clearance has been obtained from the institutional review board. Articles and any other material published in the JAFES represent the work of the author(s) and should not be construed to reflect the opinions of the Editors or the Publisher.