Conversion of Primary Hypothyroidism to Hyperthyroidism: A Case Report
Liam Clifford* and Ammar Wakil**
Liam M. Clifford, BMed Sc (Hons), BMed MD
Department of General Medicine, Wyong Hospital
Pacific Highway, Hamlyn Terrace, New South Wales 2259, Australia
Tel. No.: (02) 4394 8000
Fax No.: (02) 4394 4844
E-mail: liamclifford@live.co.uk
ORCID: https://orcid.org/0000-0002-3933-4425
e-ISSN 2308-118x
Printed in the Philippines
Copyright © 2018 by the JAFES
Received April 20, 2018. Accepted August 8, 2018.
Published Online First: November 2, 2018.
A 51-year old Caucasian male developed Graves’ thyrotoxicosis following long-standing treatment for hypothyroidism. After a short period of treatment with carbimazole, he developed agranulocytosis and required total thyroidectomy. In this relevant case report, we review several pathogenetic mechanisms that explain the transformation of autoimmune hypothyroidism into Graves’ disease and the possible approaches to the management of agranulocytosis secondary to antithyroid medications. Further studies are required to determine the best way to manage severe thyrotoxicosis when agranulocytosis develops due to antithyroid medications.
Keywords: hypothyroidism, Graves’ disease, antithyroid drugs, carbimazole, agranulocytosis
The incidence of autoimmune thyroid disease is approximately 12% in Australia, with a greater prevalence in women. Graves’ disease and Hashimoto’s thyroiditis are the most common forms of autoimmune thyroid disease.[1]
Autoimmune thyroid disease can involve one or more types of thyroid antibodies. These include the thyroid stimulating hormone (TSH) receptor antibodies, which can be divided into stimulating or blocking types. The former is thought to be the cause of Graves’ disease. Additionally, thyroid peroxidase antibody and thyroglobulin antibody are thyroid-specific antibodies commonly found in thyroid autoimmunity.[2]
It is well known that Graves’ thyrotoxicosis may be followed by hypothyroidism. However, development of Graves’ thyrotoxicosis after a period of hypothyroidism is not a common phenomenon.[3]
The treatment for Graves’ disease involves the use of antithyroid drugs such as carbimazole, methimazole and propylthiouracil. These medications are associated with several side effects, including pancytopenia and agranulocytosis. While the incidence and management of agranulocytosis is well documented, there are no clear evidence-based guidelines on the immediate management of Graves’ disease if agranulocytosis occurs. A recent study employed the use of Lugol’s solution with beta blockers to prepare patients for thyroidectomy with Graves’ disease and found this was effective.[4] In this paper, we report a case of a man who developed agranulocytosis due to carbimazole to treat Graves’ disease after long-standing primary hypothyroidism.
CASEA 51-year-old Caucasian male , was diagnosed by his General Practitioner with subclinical hypothyroidism in 2010 based on an elevated thyroid stimulating hormone level [10.7 mU/L, normal reference (NR) 0.40-3.50], normal FT4 and FT3 levels, and elevated thyroid peroxidase antibodies (604 IU/mL, NR 0-35).
His other medical conditions included hypertension, thalassemia minor, impaired glucose tolerance and cholecystitis. The patient was started on thyroxine 50 mcg daily (0.52 mcg/kg/day) , and his TSH decreased to 4.09 mU/L after 6 months and then normalized to 2.99 mU/L. After an intervening normal TSH level, indicating adequate thyroxine replacement, the patient reported weight loss and restlessness in July 2016. His biochemistry was in keeping with hyperthyroidism with suppressed TSH (<0.005 mU/L) and elevated FT4 (18.8 pmol/L, NR 9-19) and FT3 (30.5 pmol/L, NR 2.6-6 ). His thyroxine dose was reduced before being totally discontinued by his General Practitioner . Thyroid function tests at this time showed TSH <0.005 mU/L (0.40-3.50), FT4 30.5 pmol/L (NR 9-19) and FT3 19.2 pmol/L (NR 2.6-6).
After 6 months there was little change in his thyroid function tests and he was commenced on propranolol 20 mg daily and referred to a local endocrinologist. On examination, he had a diffuse, mobile goiter, blood pressure of 135/80 mm Hg and a heart rate of 75 bpm with a weight of 95 kg with a BMI of 29.3. He appeared clinically euthyroid with no signs of dermopathy, acropachy, proximal myopathy or ophthalmopathy. A thyroid ultrasound revealed heterogenous echogenicity and increased vascularity (Figure 1). A pertechnetate uptake scan showed elevated thyroid uptake, consistent with Graves’ disease (Figure 2). TSH receptor antibody (TRAb) titer was elevated at 15.3 IU/L (NR <2). Anti TPO at this time was 407.
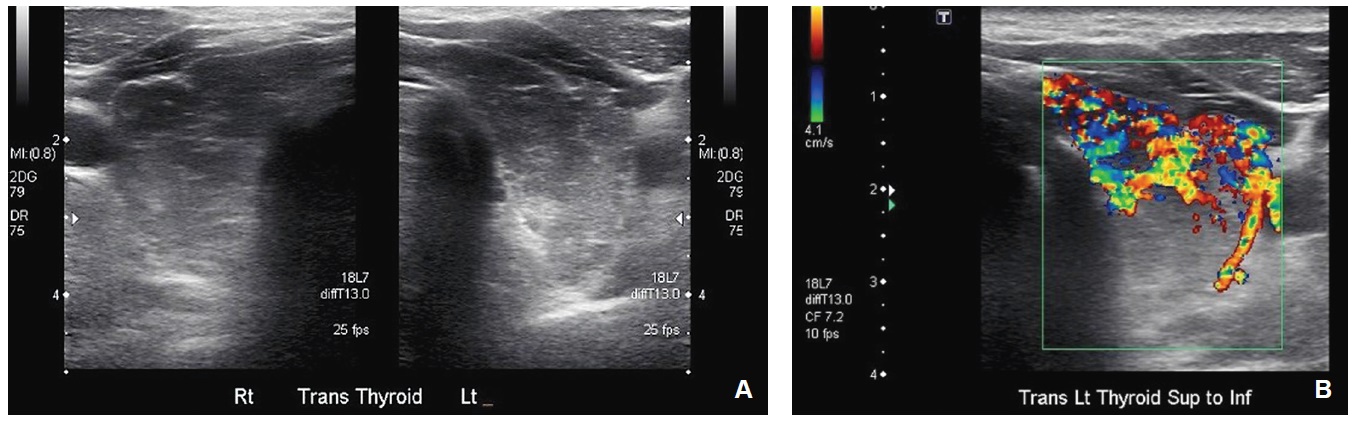
Ultrasound of the thyroid gland showing heterogenous echogenicity of the tissue parenchyma (A) and increased vascularity (B).
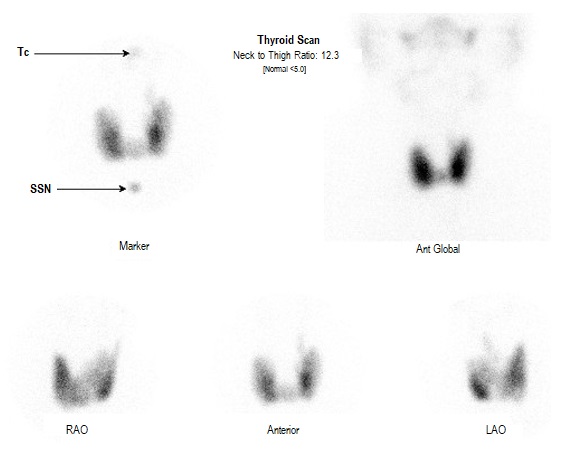
Thyroid scan showing increased pertechnetate uptake.
He was given advice on precautions regarding agranulocytosis and commenced on carbimazole 15 mg daily. After 6 weeks of treatment with carbimazole, his thyroid function tests continued to show suppressed TSH of <0.005 mU/L with raised FT4 and FT3 of 26.4 pmol/L and 12.2 pmol/L, respectively. His total daily dose of carbimazole was then increased to 25 mg. However, he was subsequently admitted to the local hospital with agranulocytosis and fever of unknown origin twenty six days after the dose of carbimazole was increased. Blood tests on admission showed white cell count (WCC) of 1.2 x10^9/L and neutrophils of 0 x 10^9/L. carbimazole was stopped and he was initially treated with filgrastim 300 mcg daily injections, intravenous cefepime 2 g QID for five days and a once-only dose of gentamicin 400 mg. Due to ongoing fever he was also commenced on vancomycin 1.5 g BID for three days concurrently. At this same time his dose of propranolol was increased to 20 mg BID. Subsequently, his neutrophil count improved on day 5. Finally, he was administered Lugol’s iodine BID for 10 days and then underwent total thyroidectomy. Histological diagnosis was Graves’ disease (Figure 3). Further analysis revealed chronic lymphocytic thyroiditis and oncocytic metaplasia (Figure 4). He is currently on thyroxine 150 mcg daily.
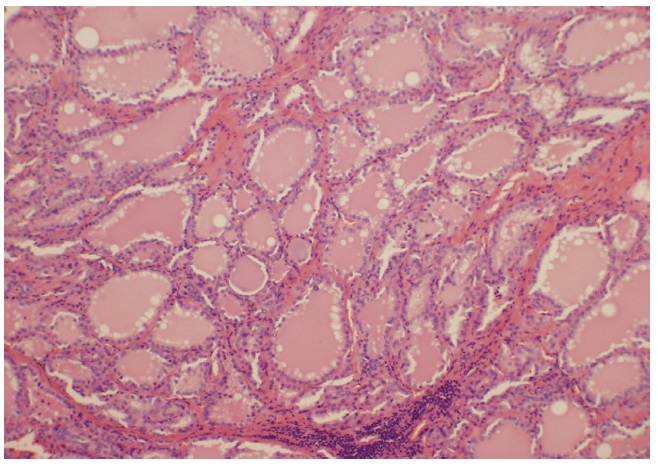
Histopathologic examination of the excised thyroid showed diffuse hyperplasia characterized by prominent scalloping in the thyroid follicles (H&E, 10x).
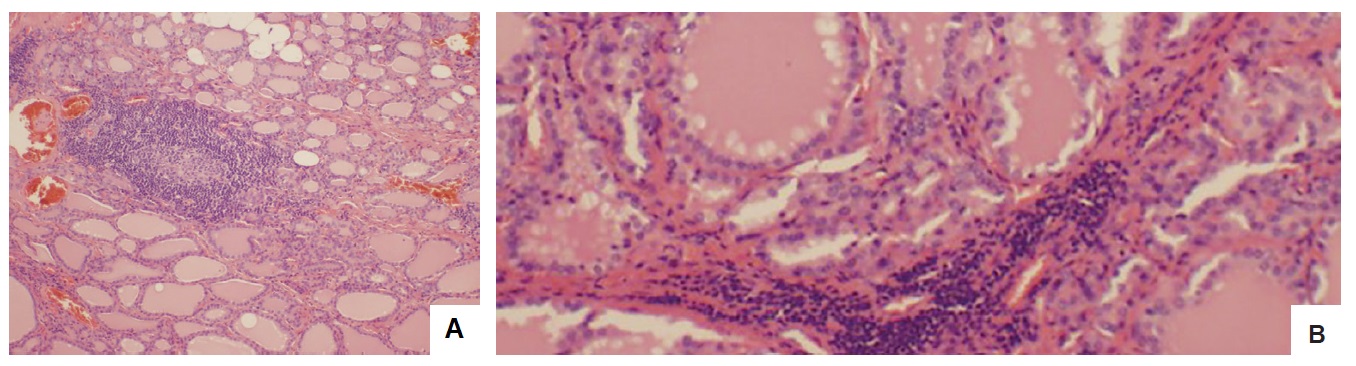
Histopathologic examination of the thyroid further revealed a background of chronic lymphocytic thyroiditis with lymphoid aggregates complete with germinal centres (H&E, 10x) (A) and oncocytic metaplasia with thyroiditis (H&E, 200x) (B).
In this case report, the patient had been on thyroxine replacement for approximately 6 years. During this period, thyroid antibodies were positive, although TSH receptor antibodies were not tested until early 2017. Our patient then went on to develop Graves’ disease. He subsequently developed agranulocytosis secondary to carbimazole, requiring treatment with Lugol’s iodine before undergoing total thyroidectomy.
The conversion of Hashimoto’s thyroiditis to Graves’ disease is documented in literature, but such cases are rare and are postulated to be due to a combination of atypical destructive thyroiditis and the development of antibodies associated with Graves’ disease.5 Several reports have suggested that Graves’ disease can follow thyroid gland destruction.[5],[6],[7],[8] One author postulated that autoimmune destruction initially produced a hypothyroid state, but the stimulatory effect of thyrotropin receptor stimulating antibodies (TSAb) and thyroid destruction may alter and subsequently create a hyperthyroid state.[5] There were also 2 studies that looked at several cases and proposed that the damage to thyroid tissue acted as the triggering factor for Graves’ disease. This also involved the production of TSH receptor antibodies which changed effects from blocking to stimulating to produce a state of hyperthyroidism.[6]-8 Another older proposed mechanism is that damage to thyroid epithelial cells leads to thyroid hormone leakage, which stimulates microsomal antigens and subsequently helper T cells to induce the production of thyroid antibodies.[7]
It is important to note that treatment with thyroxine can lead to an increase in ongoing antibody action or induce the production of TSAb in patients who may or may not have thyrotropin receptor blocking antibodies (TBAb).[9] It has been proposed that this could be due to a rise in serum T4 with replacement therapy, leading to an increased expression of stimulatory molecules that initiate antibody production. Alternatively, TBAb activity falls below the activity of TSAb, and a ‘switch’ occurs, often mediated by diminishing thyroid autoantibody levels secondary to antithyroid medications.[9]
It is well-documented in literature that the incidence rate of agranulocytosis with thionamides is 0.1-0.5%.[10] Thionamides remain the first-line treatment option for hyperthyroidism, and their mechanism of action involves inhibiting the thyroid peroxidase enzyme which decrease the production of T3 and T4.[2] The mechanism of agranulocytosis is poorly understood, with the consensus suggesting that the production of antibodies leads to an interaction with a granulocyte antigen, or causes depression of myelopoiesis.[10]
Two studies that looked at HLA profiles in the Asian population and one study in white European people concluded that some HLA genotypes were associated with antithyroid drug-induced agranulocytosis, and suggested individuals identified as carriers could potentially be offered alternative treatment at an earlier stage.[11],[12],[13] The cross-reactivity between methimazole and propylthiouracil is documented in literature, so that the use of a second antithyroid medication is contraindicated if the first antithyroid caused agranulocytosis.[14]
Most cases of agranulocytosis occur within 90 days of commencing antithyroid treatment but can occur when treatment is recommenced in the event of a relapse of hyperthyroidism.[14] The management of agranulocytosis that is induced by antithyroid medications is supportive therapy with antibiotics and G-CSF.[10]
The 3 current acceptable modalities for the treatment of hyperthyroidism are antithyroid medications, radioactive iodine and total thyroidectomy. Radioactive iodine was not selected for this patient because it can take up to 6 months before the full effect is achieved. Also, the patient was not well-controlled biochemically, even when he was taking carbimazole. The decision to undertake an urgent thyroidectomy was based on his clinical and biochemical instability. The patient was involved in this decision-making process.
One of the weaknesses in this case is that obtaining a TSAb level was not indicated at the time of diagnosis of hypothyroidism when the General Practitioner commenced thyroxine based on current guidelines of elevated thyroid peroxidase and TSH level >10 mU/L.
This case report illustrates a rare conversion of autoimmune subclinical hypothyroidism after 6 years of stable treatment with thyroxine to severe Graves’ disease. While it is unclear if TRAb was positive early in the primary hypothyroidism state, it is evident that some cases of Graves’ disease are preceded by states of hypothyroidism with TRAb present. Additionally, this case demonstrated the difficulty of treating a patient with hyperthyroidism complicated by agranulocytosis secondary to carbimazole. Further studies are required to investigate cross-reactions between antithyroid medications when agranulocytosis occurs, and other potential curative measures for hyperthyroidism following a primary hypothyroid state.
Clinicians need be aware that, albeit rare, cases of Hashimoto’s thyroiditis and hypothyroidism can convert to Graves’ thyrotoxicosis. Antithyroid drugs remain the first-line treatment option for Graves’ disease. Treatment options for thyrotoxicosis when agranulocytosis develops will need to be selected on a case-by-case basis. An experienced surgeon and an urgent thyroidectomy after a brief blockade with Lugol’s iodine was selected in our patient. This resulted in a rapid cure, in the context of clinical and biochemical instability.
AcknowledgmentsThe authors wish to thank Dr. Revadhi Chelvarajah for her assistance in performing part of the literature review and collation of data for the patient. They would also like to thank Dr. Justine Pickett for her role in the initial processing of the histopathology and Dr. Veronica Cheung for the final histopathology slides presented in this case report.
Ethical ConsiderationPatient consent was obtained before submission of the manuscript.
Statement of AuthorshipAll authors certified fulfillment of ICMJE authorship criteria.
Author DisclosureThe authors declared no conflict of interest.
Funding SourceNone.
[1] Walsh JP. Managing thyroid disease in general practice. Med J Aust. 2016;205(4):179-84. PubMed
[2] Chaudhry LA, Mazen KF, Ba-Essa E, Robert AA. Antithyroid drug induced agranulocytosis: What we still need to learn? Pan Afr Med J. 2016;23:27. PubMed PubMed Central CrossRef
[3] Lamberg BA, Salmi J, Wägar G, Mäkinen T. Spontaneous hypothyroidism after antithyroid treatment of hyperthyroid Graves’ disease. J Endocrinol Invest. 1981;4(4):399-402. PubMed CrossRef
[4] Fischli S, Lucchini B, Müller W, Slahor L, Henzen C. Rapid preoperative blockage of thyroid hormone production/secretion in patients with Graves’ disease. Swiss Med Wkly. 2016;146:w14243. PubMed CrossRef
[5] McLachlan SM. Rapoport B. Thyrotropin-blocking autoantibodies and thyroid-stimulating autoantibodies: Potential mechanisms involved in the pendulum swinging from hypothyroidism to hyperthyroidism or vice versa. Thyroid. 2013;23(1):14-24. PubMed PubMed Central CrossRef
[6] Ohye H, Nishihara E, Sasaki I, et al. Four cases of Graves’ disease which developed after painful Hashimoto’s thyroiditis. Intern Med. 2006;45(6):385-9. PubMed
[7] Umena S, Takano T. Iijima T, et al. A case of repeated painless thyroiditis followed by Graves’ disease. Endocr J. 1995;42(6):821-6. PubMed
[8] Chung YH, Ou HY, Wu TJ. Development of hyperthyroidism following primary hypothyroidism: A case report. Kaohsiung J Med Sci. 2004;20(4):188-91. PubMed CrossRef
[9] Yamasaki H, Takeda K, Nakauchi Y, Suehiro T, Hashimoto K. Hypothyroidism preceding hyperthyroidism in a patient with continuously positive thyroid stimulating antibody. Intern Med. 1995;34(4)247-50. PubMed
[10] Watanabe N, Narimatsu H, Noh JY, et al. Antithyroid drug-induced hematopoietic damage: A retrospective cohort study of agranulocytosis and pancytopenia involving 50,385 patients with Graves’ disease. J Clin Endocrinol Metab. 2012;97(1):E49-53. PubMed CrossRef
[11] Hallberg P, Eriksson N, Ibañez L, et al. Genetic variants associated with antithyroid drug-induced agranulocytosis: A genome-wide association in a European population. Lancet Diabetes Endocrinol. 2016;4(6):507-16. PubMed CRossRef
[12] Chen PL, Shih SR, Wang PW, et al. Genetic determinants of antithyroid drug-induced agranulocytosis by human leukocyte antigen genotyping and genome-wide association study. Nat Commun. 2015;6:7633. PubMed PubMed Central CrossRef
[13] Cheung CL, Sing CW, Tang CS, et al. HLA-B*38:02:01 predicts carbimazole/methimazole-induced agranulocytosis. Clin Pharmacol Ther. 2016;99(5):555-61. PubMed CrossRef
[14] Cooper DS. Antithyroid drugs. N Engl J Med. 2005;352(9):905-17. PubMed CrossRef
Authors are required to accomplish, sign and submit scanned copies of the JAFES Author Form consisting of: (1) Authorship Certification, that all the requirements for authorship have been met by each author, and that the final version of the manuscript has been read and approved by all authors; (2) the Author Declaration, that the article represents original material that is not being considered for publication or has not been published or accepted for publication elsewhere; (3) the Statement of Copyright Transfer [accepted manuscripts become the permanent property of the JAFES and are licensed with an Attribution-Share Alike-Non-Commercial Creative Commons License. Articles may be shared and adapted for non-commercial purposes as long as they are properly cited]; and the ICMJE form for Disclosure of Potential Conflicts of Interest. For original articles, authors are required to submit a scanned copy of the Ethics Review Approval of their research as well as registration in trial registries as appropriate. For manuscripts reporting data from studies involving animals, authors are required to submit a scanned copy of the Institutional Animal Care and Use Committee approval. For Case Reports or Series, and Images in Endocrinology, consent forms, are required for the publication of information about patients; otherwise, appropriate ethical clearance has been obtained from the institutional review board. Articles and any other material published in the JAFES represent the work of the author(s) and should not be construed to reflect the opinions of the Editors or the Publisher.