Single Nucleotide Polymorphism at +276 G>T of the Adiponectin Gene and Plasma Adiponectin Level in Myanmar Type 2 Diabetic Patients
Khin Thin Yu,* Kyu Kyu Maung,** Aye Thida,* Thein Myint***
Khin Thin Yu, MD
Associate Professor, University of Medicine 2
Khay Mar Thi Street, North Okkalapa Township, Yangon, Myanmar 11031
Tel. No.: +095-9690127/ 9690128/ 9690129
Fax No.:690265
E-mail: drkhinty!gmail.com
ORCID: https://orcid.org/0000-0001-9307-3898
e-ISSN 2308-118x
Printed in the Philippines
Copyright © 2018 by the JAFES
Received June 7, 2018. Accepted July 30, 2018.
Published Online First: November 27, 2018.
Objective. The aim of the study was to investigate the association between single nucleotide polymorphisms (SNP) at rs 1501299 (SNP+276 G>T) of the adiponectin gene and plasma adiponectin levels in type 2 diabetes mellitus (T2DM) patients in Myanmar.
Methodology. One hundred T2DM patients and 104 non-diabetic subjects were included in this cross-sectional analytical study. Genotype frequencies were determined by polymerase chain reaction - restriction fragment length polymorphism (PCR-RFLP) method. Plasma adiponectin level was measured by enzyme-linked immunosorbent assay (ELISA).
Results. Genotype frequencies (GG, GT, TT) of SNP+276 in diabetic patients were 39%, 48% and 13%, respectively. The GT and TT genotypes were more frequent in T2DM patients (OR 1.98, 95% CI, 1.10-3.55; p=0.02 and OR 4.07, 95% CI, 1.34-12.3; p=0.01), respectively. The T allele of SNP+276 was significantly associated with T2DM (OR 1.96, 95% CI, 1.27-3.01; p=0.002). Mean plasma adiponectin level was significantly lower than in T2DM patients (27.41±16.7 µg/mL) compared to non-diabetic subjects (37.19±26.77 µg/mL) (p=0.002).
Conclusion. SNP+276 at rs 1501299 of the adiponectin gene was associated with type 2 diabetes and low plasma adiponectin levels in this Myanmar population.
Keywords: adiponectin gene, SNP, type 2 diabetesAdiponectin is one of the most abundant proteins derived from adipose tissue. It is encoded by the adiponectin gene, located on chromosome 3q27. It has important roles in energy homeostasis, glucose and lipid metabolism, and anti-inflammatory responses in the vascular system. It is likely to modulate insulin sensitivity and to play a role in both human and animal models of insulin resistance. Insulin resistance is a fundamental element in the etiology of type 2 diabetes mellitus and is quite often associated with obesity.
The two main actions of adiponectin pertain to its insulin-sensitizing effect and anti-atherosclerotic activity. Adiponectin acts through 2 types of receptors, AdipoR-1 and AdipoR-2. AdipoR-1 is most abundantly expressed in skeletal muscle, while AdipoR-2 is found more frequently in the liver. Adiponectin decreases tissue triglyceride (TG) content and up-regulates insulin signalling via activation of peroxisome proliferator-activated receptor-α (PPAR-α) action.
Reduction of TG content in muscle is mediated by adiponectin by activation of adenosine monophosphate-activated protein kinase (AMPK). In skeletal muscle, adiponectin binds AdipoR-1 and stimulates phosphorylation of acetyl-Coenzyme A carboxylase (ACC). This leads to inhibition of ACC activity and a consequent reduction in malonyl-Coenzyme A levels, effectively depressing carnithine palmitoyl transferase-1 (CPT-1) activity and increasing fatty acid oxidation. These changes lead to decreased tissue TG content which contributes to improve insulin signal transduction.
In the liver, adiponectin binds AdipoR-2 and inhibits gluconeogenesis by AMPK-dependent phosphorylation. It decreases the expression of key enzymes involved in gluconeogenesis, such as phosphoenolpyruvate carboxykinase and glucose-6-phosphatase, effectively decreasing hepatic glucose production.
Diabetes mellitus is a multi-factorial, polygenic metabolic disorder which can affect nearly every organ system in the body. The prevalence of T2DM is increasing in South East Asia, from 39.3 million in 2003 to a projected 81.6 million in 2025. The risk of developing T2DM is determined by both genetic and environmental factors. Insulin resistance is considered to be the core factor in its pathogenesis. Genetic and epidemiological studies strongly suggest that insulin resistance is, at least in part, genetically determined.
Fifty-two candidate genes in various biochemical, regulatory and signal transduction pathways are thought to be involved in the pathogenesis of T2DM. Adiponectin is one such gene.[1] Genome-wide scans in humans have mapped a susceptibility locus for T2DM and the metabolic syndrome to chromosome 3q27, where the gene encoding adiponectin is also located. It spans 17 kilo bases and consists of 3 exons and 2 introns.[2]
Genetic variations in the adiponectin gene can affect plasma adiponectin concentration. It is estimated that a 30 to 70% variation in normal circulating adiponectin level can be attributed to genetic factors. Serum concentrations of adiponectin are heritable, making it a strong candidate gene for T2DM, obesity and coronary artery disease (CAD).[3] A total of 42 single nucleotide polymorphisms in the adiponectin gene and its regulatory region with a minor allele frequency of more than 1.5% have been identified.
Many studies investigating the association of genetic variations in the adiponectin gene with plasma adiponectin level and T2DM have recently been published.[4] The association between adiponectin gene polymorphisms and plasma adiponectin level in T2DM has been demonstrated in various studies on Asian populations and Western populations. The G>T polymorphism of SNP+276 in intron 2 of the adiponectin gene has been found to be related to type 2 diabetes in Japanese subjects, Iranian obese individuals and in non-diabetic Greek women.[3],[5],[6]. Adiponectin gene polymorphisms affect the development of diabetes, obesity and insulin resistance, and are influenced by differences in genetic background and environmental factors in various ethnic populations.[7]
There are 3 forms of genotype distribution (GG, GT, TT) at rs 1501299 (+276 G>T) of the adiponectin gene, when the base guanine (G) changes to thymine (T). This SNP occurs when the normal wild type G allele is changed into a T allele (GT or TT). This was found to be associated with an increased risk for T2DM. Moreover, because hypoadiponectinemia has been strongly linked to obesity, insulin resistance and T2DM, it may be used to predict the overall risk of developing insulin resistance and overt T2DM. Low serum adiponectin was associated with impaired glucose tolerance and T2DM in a Finnish population.[8] Plasma adiponectin was determined to be an independent predictor of T2DM in Asian Indian and Jordanian study populations.[9], [10]
The association between plasma adiponectin level and SNP+276 G>T of the adiponectin gene in T2DM has subsequently been found in various ethnic groups such from Japan, China, Korea, India, Malaysia, Egypt, and also those of Caucasian, African American origin. Although not consistent, many studies have also found the association between this genetic variation and plasma adiponectin level in different study groups. This study will investigate the genetic variation of the adiponectin gene and determine the association between SNP+276 G>T and its gene product, adiponectin, in a population from Myanmar, in relation to T2DM.
METHODOLOGYStudy population
This cross-sectional, analytical study recruited patients with T2DM from the out-patient department and diabetes clinic of North Okkalapa General Hospital. Non-diabetic subjects were selected by simple random sampling from Quarter B, North Okkalapa Township, Yangon, Myanmar. The randomly selected subjects with fasting plasma glucose (FPG) less than 6.1 mmol/L (110 mg/dL) were considered as non-diabetic, based on the World Health Organization 2006 criteria.[11]
Study procedure
A sample of 5 mL venous blood was taken from all subjects for the determination of plasma adiponectin levels and genotyping. Separation of plasma from the blood sample was done by centrifugation at 1500 rpm for 20 minutes. Deoxyribonucleic acid (DNA) extraction was done on the day of sample collection at the Common Research Laboratory, University of Medicine 2, Yangon. The extracted DNA were stored as dry form at -20°C. The remaining plasma samples were stored at -20°C in deep freeze for determination of adiponectin and genotyping. These were carried out within 6 months of sample collection.
Determination of plasma glucose level was done by enzymatic colorimetric test (Human GmBH-Germany). Determination of plasma adiponectin level was done by ELISA method (DRG International, Inc., USA).
DNA extraction was done by salting out method. Purity of DNA was checked by agarose gel electrophoresis. Specific DNA fragments consisting of SNP+276 was amplified from genomic DNA by specific primer set and the products were identified in 2% agarose gel and seen at 241 bp. These PCR products were digested with restriction enzyme (BsmI) (New England BiolabsⓇ, USA) and the products were separated by 2% agarose gel to analyze for RFLP. Three different genotypes were noted. The following primers were used for SNP+276 genotyping by PCR-RFLP :

Statistical analysis
Plasma adiponectin levels were expressed as means and standard deviations. Genotype and allele frequencies were expressed as percentages. Mean plasma adiponectin levels were compared across genotypes using ANOVA and Tukey HSD test, and between T2DM and non-diabetic subjects by Student’s t-test. Hardy-Weinberg equilibrium and the association between disease status and the genetic variants were tested by Pearson’s Chi-square test. Odds ratios, 95% confidence intervals and all statistical tests were carried out using SPSS® software v. 16.0.
Ethical considerations
The research was done according to the international ethical guidelines of the Council for International Organization of Medical Science. Ethical approval was obtained from the Ethical Review Committee of University of Medicine 2, Yangon.
A total of 204 participants consisting of 100 patients with T2DM and 104 non-diabetic subjects fulfilled the inclusion criteria and were accounted for analysis in this study. The age range in this population was 35 to 65 years old. Although majority of the participants were female, there was no significant difference in sex distribution between 2 groups of participants (p=0.41).
Mean body mass index was significantly higher in patients with T2DM (24.81±5.23) compared to non-diabetic subjects (23.53±3.88) (p<0.05).
Figure 1A shows the PCR products of adiponectin gene. Specific DNA fragments consisting SNP+ 276 were amplified from genomic DNA by a specific primer set. The products were then identified in 2% agarose gel and seen at 241 bp. These PCR products were digested with BsmI. The digested products were separated by 2% agarose gel to analyze for RFLP.
Three different genotypes were noted (Figure 1B). Lane 2 showed homozygous wild (GG) genotype, seen as 2 bands at 95 bp and 146 bp. At Lane 5, heterozygous (GT) genotype was seen as 3 bands at 95 bp, 146 bp and 241 bp. Lane 1 showed homozygous mutant (TT) genotype which was seen as a single band at 241 bp.
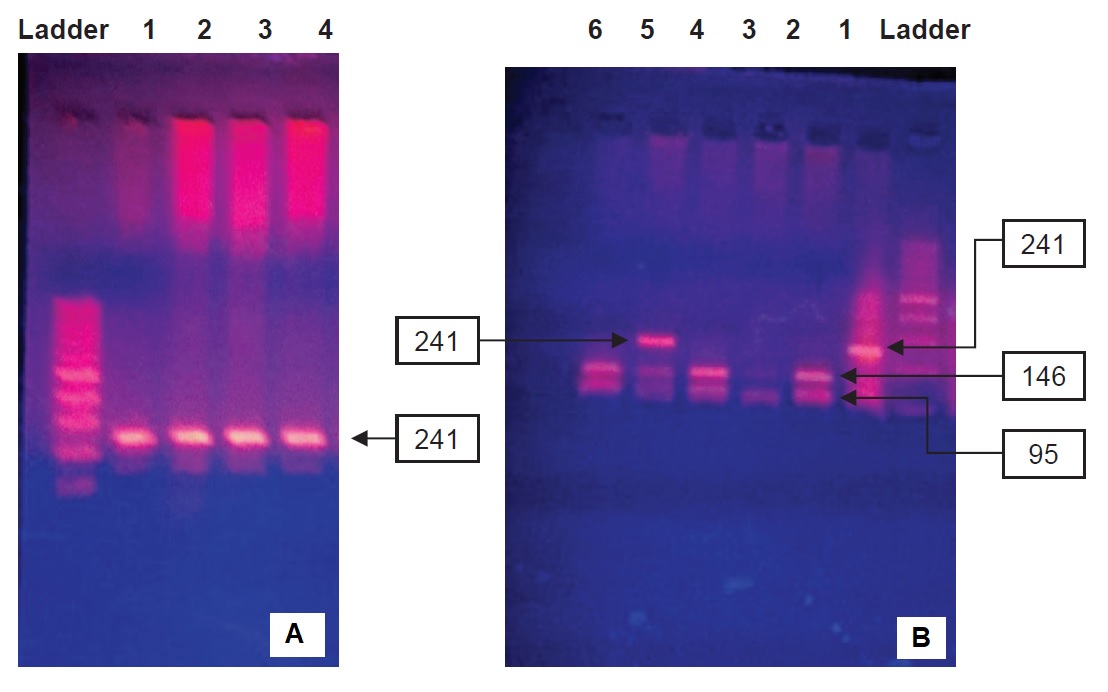
Figure 1. Restriction enzyme digests on 2% agarose gel. A PCR products of SNP+276. B genotyping of SNP+276.
A one-way ANOVA between subjects was conducted to compare the plasma adiponectin levels with different genotypes (GG, GT and TT) (Table 1). Post hoc comparisons using the Tukey HSD test indicated that there was a statistically significant difference between the mean adiponectin level of patients with GG genotype (31.64±18.95) compared to TT (19.77 + 11.85) (Table 2).
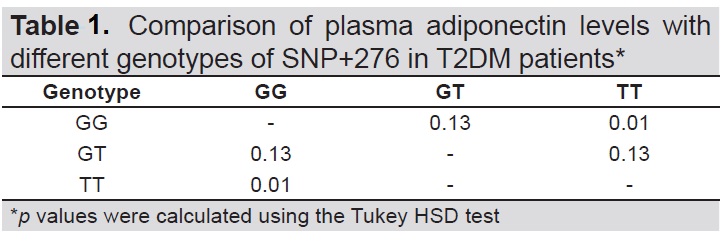
Table 1. Comparison of plasma adiponectin levels with different genotypes of SNP+276 in T2DM patients*
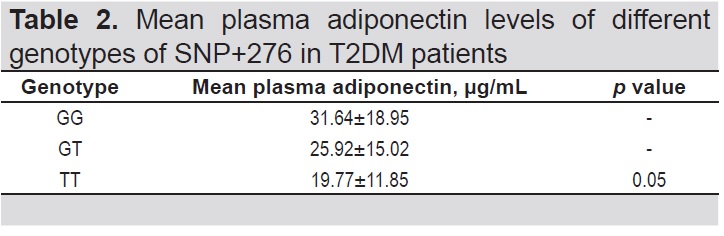
Table 2. Mean plasma adiponectin levels of different genotypes of SNP+276 in T2DM patients
Type 2 diabetes mellitus is one of the most common metabolic diseases and poses a substantial burden on health care systems globally. There is compelling data that genetic susceptibility to T2DM is polygenic. Genome-wide association studies have identified almost 50 loci associated with T2DM risk. Adiponectin gene polymorphism may play a causal role in the pathogenesis of insulin resistance and T2DM.[12]
Among the adiponectin gene SNPs, the intronic SNP+276 G>T at rs 1501299 is considered the important known genetic risk factor for the development of insulin resistance and T2DM. Since adiponectin regulates both glucose and lipid metabolism, derangement of these processes due to reduced adiponectin levels will lead to insulin resistance and subsequent T2DM. Many studies have demonstrated the association of reduced plasma adiponectin levels with T2DM. The aim of the present study was to provide supportive evidence for the involvement of the adiponectin gene and its effect on plasma adiponectin levels in T2DM patients from Myanmar.
In this study, SNP+276 G>T of heterozygous (GT) and homozygous (TT) genotypes were significantly more frequent in T2DM patients than in non-diabetic subjects. These are consistent with findings of greater frequency of GT and TT in obese T2DM patients from Saudi Arabia, South India and Finland[13],[14],[15] The genotype frequencies in this study (GG 39.4%, GT 48.1% and TT 12.5%) are closest to the findings in Finland (GG 45%, GT 43% and TT 12%), and are comparable with distribution frequencies worldwide.[15] The genotype distribution of SNP +276 conformed to the Hardy-Weinberg equilibrium principle in both the T2DM (Ӽ2=0.41, df =1, p=0.522) and non-diabetic groups (Ӽ2=0.67, df=1, p=0.413). Since the comparison was made in patients with T2DM and non-diabetic subjects, the genotypes GT and TT do not give a greater risk for DM but is associated with the presence of T2DM OR 1.98, 95% CI, 1.10-3.55, p=0.02 and OR 4.07, 95% CI, 1.34-12.3, p=0.01, respectively) (Table 3). These results were similar to the findings in a study of Taiwanese diabetic patients which demonstrated that T2DM was more common in subjects with GT and TT genotypes.[7]
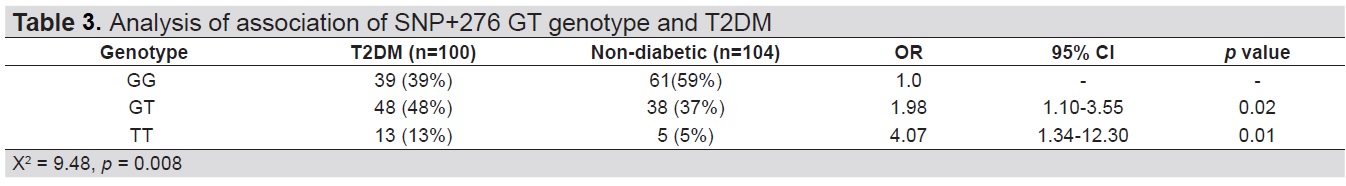
Table 3. Analysis of association of SNP+276 TT genotype in T2DM
Analysis of allele frequencies of SNP+276 showed that the risk allele T was found more frequently in T2DM patients compared to non-diabetic subjects (OR 1.96, 95% CI, 1.27-3.01, p=0.002) (Table 4). The higher risk of developing T2DM in T allele carriers was seen in studies on Taiwanese and Saudi Arabian populations.[13],[16] These support the possible susceptibility role of the T allele of SNP+276 for T2DM. This polymorphism might be a predisposing factor to T2DM in the Myanmar population.
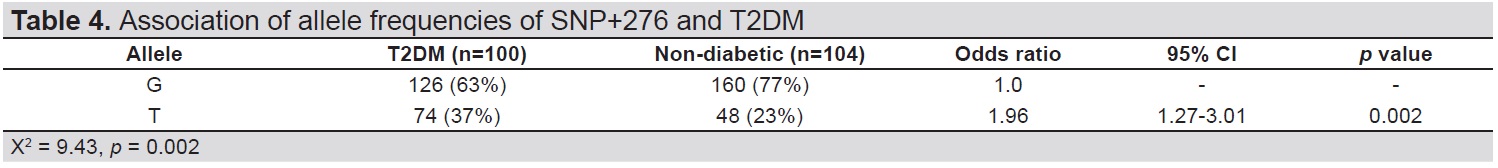
Table 4. Association of allele frequencies of SNP+276 and T2DM
In the current study, the mean plasma adiponectin level of T2DM patients (27.41±16.7 µg/mL) was significantly lower compared to the non-diabetic subjects (37.19±26.77 µg/mL). Mean plasma adiponectin levels of GT and TT genotypes were also lower than that of GG. There was a statistically significant difference in the mean plasma adiponectin levels of GG and TT genotypes. These were consistent with studies on Japanese and Asian Indian populations, and in a meta-analysis including 13 prospective studies of Caucasian, Asian Indian, African and native American ethnic groups.[9],[17],[18] SNP+276 G>T appears to influence plasma adiponectin levels and subsequent development of T2DM. While the relatively small study population may not represent the whole Myanmar population, the results remain relevant in the analysis of various ethnic groups and adiponectin gene polymorphisms.
There is no general agreement regarding the mechanism of SNP+276 and observed decreased plasma adiponectin levels. Because SNP+276 is situated in intron 2 of the adiponectin gene away from the consensus splice site, it does not have a known function. It may be a marker of some other variants that affecting adiponectin gene expression. It has been demonstrated that the SNP+276 G>T is in almost complete linkage disequilibrium with several polymorphisms placed in the 3’ untranslated regions (3’UTR).[19] 3’UTR is a region with a pivotal role in the control of gene expression by binding proteins that regulate mRNA processing, translation or degradation.
Insulin sensitizer, a PPAR-ϒ agonist thiazolidinediones, have been shown to increase plasma adiponectin levels in mice and humans. Novel therapeutic strategies for T2DM may then involve up-regulation of adiponectin receptors and stimulation of adiponectin receptors using small molecule agonists.
Study limitations
This study was able to demonstrate the interesting association between the SNP+276 and T2DM and its effect on plasma adiponectin levels. One of the limitations was the use of Fasting plasma glucose only to exclude diabetes mellitus, instead of the standard 75 g oral glucose tolerance test and glycosylated haemoglobin (HbA1c). This poses the possibility of inclusion of diabetic patients who may only have elevated 2-hour post-load glucose or high HbA1C in the non-diabetic group. Moreover, the correlation between plasma adiponectin level and insulin resistance was not included in this study. Other various confounding factors and their covariate effects were not considered during the analysis of the association between SNP and presence of T2DM.
The findings demonstrated that the odds of having type 2 diabetes was increased four-fold in the presence of the T allele of SNP +27. Genotypes GG and GT were associated with lower plasma adiponectin levels. The current study provided additional evidence for the potential involvement of the adiponectin gene as a risk factor for T2DM in the Myanmar population. It may be recommended that the non-diabetic subjects who had risk alleles of SNP+276 and their family members undergo periodic screening for diabetes.
AcknowledgmentsThe authors would like to express their deep gratitude to the Rector, University of Medicine 2, Yangon for the approval to carry out the study, and to the staff of the Biochemistry Department, University of Medicine 2, Yangon, for their kind cooperation.
Statement of AuthorshipAll authors certified fulfillment of ICMJE authorship criteria.
Author DisclosureThe authors declared no conflict on interest.
Funding SourceNone.
[1] Kaput J, Noble J, Hatipoglu B, Kohrs K, Dawson K, Bartholomew A. Application of nutrigenomic concepts to type 2 diabetes mellitus. Nutr Metab Cardiovasc Dis. 2007;17(2):89-103. PubMed CrossRef
[2] Li LL, Kang XL, Ran XJ, et al. Associations between 45T/G polymorphism of the adiponectin gene and plasma adiponectin levels with type 2 diabetes. Clin Exp Pharmacol Physiol. 2007;34(12):1287-90. CrossRef
[3] Hara K, Boutin P, Mori Y, et al. Genetic variation in the gene encoding adiponectin is associated with an increased risk of type 2 diabetes in the Japanese population. Diabetes. 2002;51(2):536-40. PubMed
[4] Kadowaki T, Yamauchi T, Kubota N, Hara K, Ueki K, Tobe K. Adiponectin and adiponectin receptors in insulin resistance, diabetes, and the metabolic syndrome. J Clin Invest. 2006;116(7):1784-92. PubMed PubMed Central CrossRef
[5] Mohammadzadeh G, Zarghami N. Associations between single-nucleotide polymorphisms of the adiponectin gene, serum adiponectin levels and increased risk of type 2 diabetes mellitus in Iranian obese individuals. Scand J Clin Lab Invest. 2009;69(7):764-71. PubMed CrossRef
[6] Melistas L, Mantzoros CS, Kontogianni M, Antonopoulou S, Ordovas JM, Yiannakouris N. Association of the +45 T>G and +276 G>T polymorphisms in the adiponectin gene with insulin resistance in nondiabetic Greek women. Eur J Endocrinol. 2009;161(6):845-52. PubMed PubMed Central CrossRef
[7] Yang WS, Yang YC, Chen CL, et al. Adiponectin SNP276 is associated with obesity, the metabolic syndrome, and diabetes in the elderly. Am J Clin Nutr. 2007;86(2):509-13. PubMed CrossRef
[8] Jalovaara K, Santaniemi M, Timonen M, et al. Low serum adiponectin level as a predictor of impaired glucose regulation and type 2 diabetes mellitus in a middle-aged Finnish population. Metabolism. 2008;57(8):1130-4. PubMed CrossRef
[9] Snehalatha C, Mukesh B, Simon M, Viswanathan V, Haffner SM, Ramachandran A. Plasma adiponectin is an independent predictor of type 2 diabetes in Asian Indians. Diabetes Care. 2003;26(12):3226-9. PubMed
[10] Aleidi S, Issa A, Bustanji H, Khalil M, Bustanji Y. Adiponectin serum levels correlate with resistance in type 2 diabetic patients. Saudi Pharm J. 2015;23(3):250-6. PubMed PubMed Central CrossRef
[11] World Health Organization and International Diabetes Federation. Definition and diagnosis of diabetes mellitus and intermediate hyperglycemia: Report of a WHO/IDF consultation. Geneva, Switzerland: World Health Organization, 2006. http://apps.who.int/iris/handle/10665/43588
[12] Sladek R, Rocheleau G, Rung J, et al. A genome-wide association study identifies novel risk loci for type 2 diabetes. Nature. 2007;445(7130):881-5. PubMed CrossRef
[13] Mackawy AMH, Alzohairy MAA, Ahmed EAA, Badawy MEH. Adiponectin gene polymorphism and the incidence of type 2 diabetes mellitus in obese patients in Qassim Region, Saudi Arabia. J Am Sci. 2011;7(12):432-43.
[14] Thirunavukkarasu A, Nithya R, Muthukumaran K, Sivasankari C. Association of the 45 T/G and 276 G/T polymorphisms in the adiponectin gene with type 2 diabetes in South Indian population. J Environ Res Develop. 2014;8(3A):563-7.
[15] Siitonen N, Pulkkinen L, Lindström J, et al. Association of ADIPOQ gene variants with body weight, type 2 diabetes and serum adiponectin concentrations: The Finnish Diabetes Prevention Study. BMC Med Genet. 2011;12:5.PubMedPubMed Central CrossRef
[16] Huang MC, Wang TN, Lee KT. Adiponectin gene SNP276 variants and central obesity confer risks for hyperglycemia in indigenous Taiwanese. Kaohsiung J Med Sci. 2010:26(5):227-36. PubMed CrossRef
[17] Hotta K, Funahashi T, Arita Y, et al. Plasma concentrations of a novel, adipose-specific protein, adiponectin in type 2 diabetic patients. Arterioscler Thromb Vasc Biol. 2000;20(6):1595-9. PubMed
[18] Li S, Shin HJ, Ding EL, van Dam RM. Adiponectin levels and risk of type 2 diabetes: A systematic review and meta-analysis. JAMA. 2009;302(2):179-88. PubMed CrossRef
[19] Menzaghi C, Ercolino T, Di Paola R, et al. A haplotype at the adiponectin locus is associated with obesity and other features of the insulin resistance syndrome. Diabetes. 2002;51(7):2306-12. PubMed
Authors are required to accomplish, sign and submit scanned copies of the JAFES Author Form consisting of: (1) Authorship Certification, that all the requirements for authorship have been met by each author, and that the final version of the manuscript has been read and approved by all authors; (2) the Author Declaration, that the article represents original material that is not being considered for publication or has not been published or accepted for publication elsewhere; (3) the Statement of Copyright Transfer [accepted manuscripts become the permanent property of the JAFES and are licensed with an Attribution-Share Alike-Non-Commercial Creative Commons License. Articles may be shared and adapted for non-commercial purposes as long as they are properly cited]; and the ICMJE form for Disclosure of Potential Conflicts of Interest. For original articles, authors are required to submit a scanned copy of the Ethics Review Approval of their research as well as registration in trial registries as appropriate. For manuscripts reporting data from studies involving animals, authors are required to submit a scanned copy of the Institutional Animal Care and Use Committee approval. For Case Reports or Series, and Images in Endocrinology, consent forms, are required for the publication of information about patients; otherwise, appropriate ethical clearance has been obtained from the institutional review board. Articles and any other material published in the JAFES represent the work of the author(s) and should not be construed to reflect the opinions of the Editors or the Publisher.