Validity and Reliability of the European Organization for Research and Treatment of Cancer Quality of Life Questionnaire Core 30 – Tagalog among Adult Filipinos with Differentiated Thyroid Cancer*
Diane Carla Bernardo, Ralph Jason Li, Cecilia Jimeno
Diane Carla C. Bernardo, MD
Section of Endocrinology, Diabetes and Metabolism
Department of Medicine, University of the Philippines-Philippine General Hospital
Taft Avenue, Ermita, Manila, Philippines 1000
Tel. No.: +632-554-8400 local 2230
E-mail: dcbeemd@gmail.com
ORCID: https://orcid.org/0000-0002-7537-6681
e-ISSN 2308-118x
Printed in the Philippines
Copyright © 2018 by the JAFES
Received July 4, 2018. Accepted July 31, 2018.
Published Online First: September 13, 2018.
*Data from this study has been presented at the Philippine Society of Endocrinology, Diabetes and Metabolism (PSEDM) convention as part of the Philippine Research Initiative on Diabetes and Endocrinology session at the EDSA Shangrila Manila, on March 22, 2018, and at the Seoul International Congress of Endocrinology and Metabolism (SICEM) poster session at the Grand Walkerhill Seoul, South Korea, on April 20, 2018.
Objective. This study aims to determine the convergent and discriminant validity and internal consistent reliability of the European Organization for Research and Treatment of Cancer Quality of Life Questionnaire Core 30 (EORTC QLQ-C30) Tagalog among adult Filipinos with differentiated thyroid cancer (DTC).
Methodology. 104 adult Filipinos with DTC at various disease stages self-administered the EORTC QLQ-C30 version 3 Tagalog and Short Form-36 (SF-36) version 2 Tagalog. Concurrent validity between conceptually-related scales from both tools was determined. Convergent and discriminant validity of multi-item scales of the EORTC QLQ-C30 Tagalog were assessed by Spearman’s correlation. Cronbach's α was computed.
Results. The EORTC QLQ-C30 Tagalog showed moderate correlation with similar scales in the SF-36 Tagalog particulary for physical, role and social functioning, pain, and global health (r=0.42-0.48, p<.001). It showed satisfactory item-domain convergent and discriminant validity for all scales except pain, fatigue, physical and cognitive functioning. Internal consistent reliability was good with cronbachs α ranging from 0.77 to 0.88 for global health, emotional and role functioning and symptom scale of nausea/vomiting.
Conclusion. The EORTC QLQ-C30 Tagalog had acceptable convergent and discriminant validity and internal consistent reliability for the scales of global health, role, social and emotional functioning and nausea/vomiting when applied among adult Filipinos with DTC.
Keywords: thyroid neoplasms, quality of life, validation studies, EORTC QLQ-C30An assessment of disease and treatment outcomes of any patient with cancer is usually incomplete without an evaluation of health-related quality of life (HRQoL). In recent years, there has been a growing body of literature focusing on this outcome measure not only among patients with more common cancers like breast and colon, but also others including thyroid.[1]
In the Philippines, thyroid cancer is ranked eighth of the top ten cancers, with an estimate of 3288 new cases among men and women in 2015.[2] In tertiary centers with facilities for total thyroidectomy and radioactive iodine (RAI), mortality rates are low at 0.3% for papillary thyroid cancer and 2.5% for follicular thyroid cancer.[3] While increased survival and good response to treatment are established among Filipino thyroid cancer patients, there are currently no published studies about the HRQoL in this specific population.
One reason for the paucity of HRQoL-related research on thyroid cancer in the Philippines is the limited number of validated tools in Tagalog to assess different quality of life dimensions. The European Organization for Research and Treatment of Cancer Quality of Life Questionnaire Core 30 (EORTC QLQ-C30) is one of the most widely used tools for quality of life assessment initially developed in Europe and used in around 3000 studies worldwide since its development.[4] It is a general cancer questionnaire, with its latest version (version 3) having been translated in Tagalog and pre-tested among 15 Filipino patients with different cancers as per procedure from the EORTC Quality of Life Group.[5]-[6] The psychometric properties of the Tagalog version have been previously evaluated in a sample of adult Filipino females with breast cancer but not in thyroid cancer patients.[7]
Recently, a thyroid cancer-specific module in Tagalog was also developed and validated to be used alongside the pre-tested EORTC QLQ-C30 Tagalog.[8] Application of the two questionnaires on patients with differentiated thyroid cancer (DTC) during the module development process revealed low correlation between scales from the two tools suggesting each tool reveals unique aspects in HRQoL and thus may be administered together.[8]
This study was done in order to evaluate the convergent and discriminant validity. concurrent validity, as well as internal consistent reliability of the EORTC QLQ-C30 Tagalog before clinical application in larger scale studies on HRQoL among Filipino patients with DTC can be initiated.
METHODOLOGYSetting
The Philippine General Hospital outpatient clinics (medicine, surgery, thyroid) was the study site for patient recruitment. It is a tertiary hospital in Manila catering to around 10,000 patients with benign and thyroid disease per year and referred from across the Philippines.[9]
Study design and sample size
This cross sectional-analytical study was part of a larger study seeking to determine factors affecting quality of life among adult Filipinos with differentiated thyroid cancer who have undergone thyroidectomy with or without RAI (Protocol No 2017-296-01).
A sample size of 96 subjects each responding to 30 items achieves 80% power to detect the difference between the coefficient alpha under the null hypothesis of 0.70000 and the coefficient alpha under the alternative hypothesis of 0.80000 using a two-sided F-test with a significance level of 0.05000. With a 10% allowance for non-response, a total of 106 subjects were recruited for the study.
106 adult Filipinos age 19 or above, with histopathologic-confirmed diagnosis of DTC at least two months after any form of thyroidectomy and/or RAI, at any disease stage, and who can understand and read Tagalog consecutively attending the outpatient clinics were recruited. Respondents with acute illness or chronic illness in acute decompensation, with concomitant malignancy, cognitive impairment or severe eye disease precluding questionnaire completion were excluded. After giving written informed consent, all eligible patients answered two questionnaires, the EORTC QLQ-C30 Tagalog version 3 and the Short Form 36 Tagalog version 2 (SF-36), the reference used to evaluate concurrent validity. All questionnaires were self-administered after appropriate instructions from research assistants, who were available to answer any questions before and until the patient completed the forms. Respondents answered questionnaires in a quiet room before or after seeing their attending with no time limits imposed.
Medical charts were reviewed for clinical data and interviews were done to complete information on demographics not available in the chart. Thyroid cancer staging was based on the American Joint Committee on Cancer (AJCC) classification system, 7th edition, for differentiated thyroid carcinoma.[10] Response to Therapy was based on recommendations from the 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer.[11]
Study tools
A. European Organization for Research and Treatment of Cancer Quality of Life Questionnaire Tagalog
The EORTC QLQ-C30 version 3 Tagalog is a thirty-item general cancer questionnaire consisting of one global health scale, five functional domain scales (physical, social, role, cognitive, emotional), and several cancer-related symptoms (fatigue, nausea/vomiting, dyspnea, insomnia, appetite loss, constipation, diarrhea, and financial difficulties). Responses to questions under the five functional domains and symptoms are rated on a four-point scale, from “not at all” to “very much”, while responses to questions under global quality of life are rated on a seven-point scale from “very poor” to “excellent”. All domain and item scores were linearly transformed into a scale of 0 to 100 as per EORTC Scoring Manual.[12] A high score for a functional domain and global health scale means a relatively high level of functioning while a high score for each symptom means a higher level of problems.
B. Short form-36 Tagalog
The SF-36 is a thirty-six-item questionnaire that evaluates general health status across any population, with disease or well. It assesses domains such as physical health, role limitation due to physical or emotional problems, bodily pain, vitality, social functioning, mental functioning and general health. Items are scored on a three or five-point scale depending on the domain. Raw scores are transformed into a 0-100 scale as per developers’ scoring instructions. Higher scores mean better level of functioning and quality of life.
The second version of the SF-36 was used as the reference to evaluate concurrent validity with the EORTC QLQ-C30 Tagalog as the former had been previously translated in Tagalog and validated among a large sample of community and urban-dwelling Filipinos.[13] Furthermore, it is comprehensive and evaluates similar dimensions in quality of life with the EORTC QLQ- C30.
Ethical consideration
Ethical clearance was obtained from the University of the Philippines Manila Research Ethics Board. Permission to use EORTC QLQ-C30 and SF-36 Tagalog versions were obtained from their respective developers.
Statistical analysis
Descriptive statistics were used to summarize the clinical and demographic characteristics of the respondents. Frequency and proportion were used for nominal variables, median and range for ordinal variables, and mean and standard deviation for interval/ratio variables. Spearman’s correlation coefficient was computed to measure concurrent validity between homologous scales of the EORTC QLQ-C30 and SF-36, as well as to correlate the scores of individual items to its own domain and other domains for convergent and discriminant validity respectively. Convergent validity was satisfied if item-domain correlation was >0.45 while discriminant validity was satisfied if the correlation coefficient between an item with its own domain was higher than other domains[14] Scaling success rate for convergent validity was computed as the percentage of item to within-scale correlations >0.45 over total number of correlations. Scaling success rate for discriminant validity was computed as the percentage of items with correlations that are higher within-scale than with other scales over total number of correlations.
Cronbach’s α was used to measure internal consistent reliability of the domains of the EORTC QLQ C-30 Tagalog. Internal consistent reliability was met if α is at least 0.70.[14]
Data were encoded using Microsoft Excel and analyzed using STATA 15.
A total of 104 respondents were included in the final analysis. Two patients were excluded: one due to an extensive number of missing answer to items (>50%) while the other due to the presence of another malignancy aside from DTC on chart review (Figure 1).
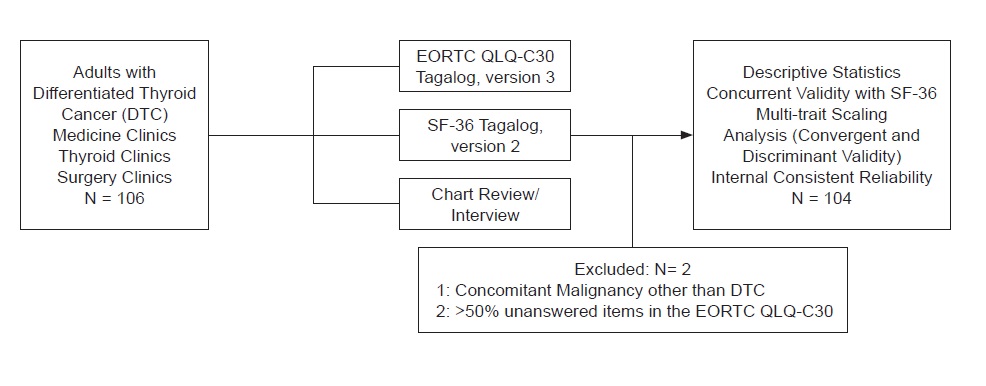
Figure 1. Participant flow.
The mean age of respondents was 43 years. Many were from Metro Manila (40%), and Region 4 (32%), Region 3 (17%), which are Tagalog-speaking provinces. Majority were female (84%) and finished high school (58%), were married (54%) and unemployed (66%). The most common type of cancer was papillary thyroid cancer and its variants, which is consistent with national figures.2 Majority also underwent total thyroidectomy (77%) without neck dissection (75%) and received RAI (70%) at different doses (Table 1). Respondents took about an average of nine minutes (range 5-25) to accomplish the EORTC QLQ-C30 Tagalog and twenty minutes (range 5-56) to accomplish the SF-36 Tagalog.

Table 1. Distribution of respondents according to clinical and demographic characteristics (N=104)
Correlation between homologous scales of EORTC QLQ-C30 Tagalog and SF-36 Tagalog was moderate particularly in the scales of physical functioning, role functioning, pain, global health and social functioning. Weak correlation was found in the scales of fatigue and vitality as well as emotional functioning and mental health (Table 2).
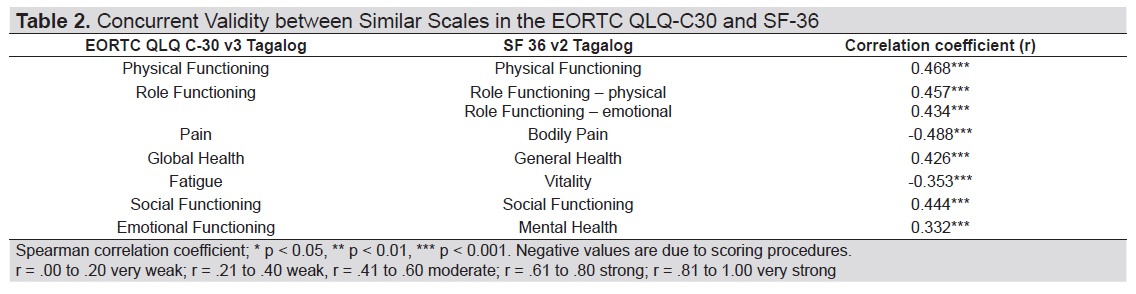
Table 2. Concurrent validity between similar scales in the EORTC QLQ-C30 and SF-36
Multi-trait scaling analysis of the Tagalog version of the EORTC QLQ-C30 showed satisfactory convergence particularly for the scales of global health, role functioning, emotional functioning, social functioning and the symptom scale of nausea/vomiting (Table 3 and Table 4), where correlation of items with their own domain was >0.45. Discriminant validity was also demonstrated for the same aforementioned domains save for item 26 under social functioning which correlated more with the role functioning scale. Convergent and discriminant validity criteria were not satisfied for physical functioning, cognitive functioning, fatigue and pain scales.
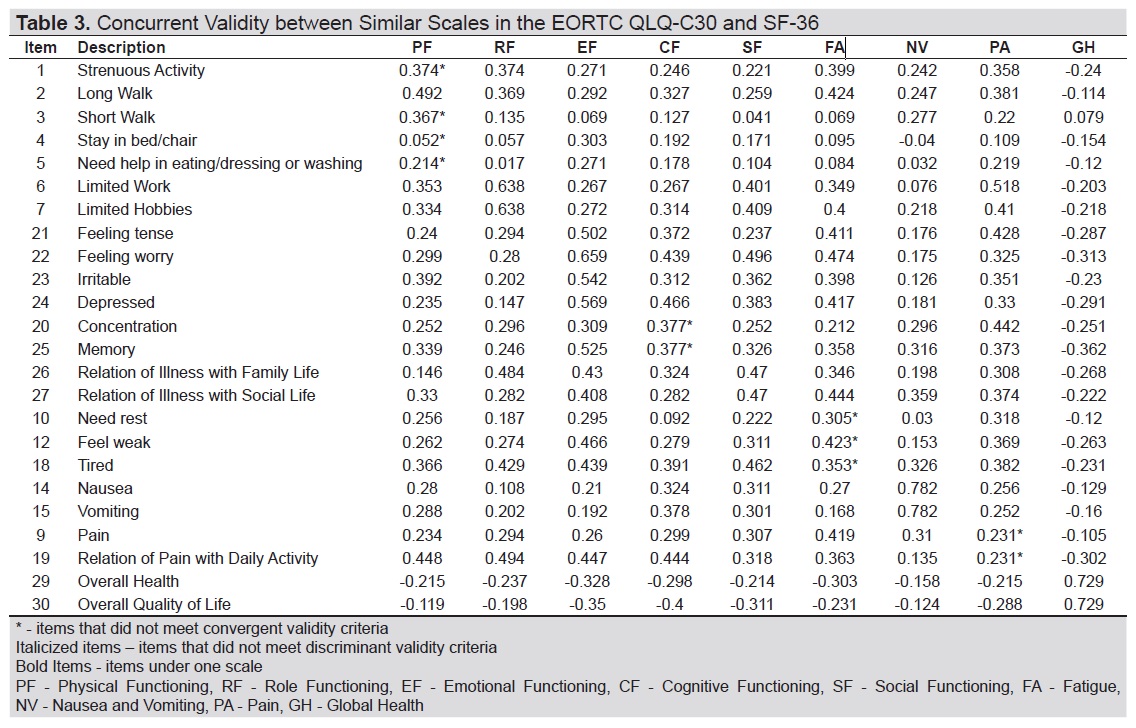
Table 3. EORTC QLQ-C30 Tagalog: Item-scale correlation matrix

Table 4. Convergent and discriminant validity for multi-item scales of the EORTC QLQ-C30 Tagalog
Internal consistent reliability was demonstrated for the Tagalog version of the EORTC QLQ-C30 for the scales global health, role functioning, emotional functioning and symptom scale of nausea and vomiting (Table 5). Overall cronbach’s α for functional domains was 0.72 and 0.78 for symptom scales.
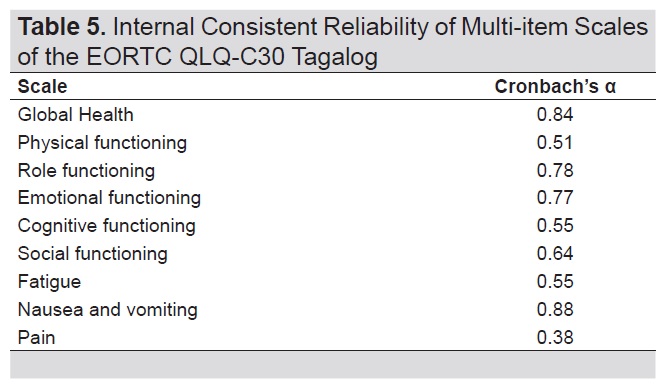
Table 5. Internal consistent reliability of multi-item scales of the EORTC QLQ-C30 Tagalog
The few existing studies on health-related quality of life among cancer patients in the Philippines mostly enroll patients with more prevalent cancers or those with terminal cancers using the EORTC QLQ-C30 Tagalog.[7],[15]-[16] None have been done on emerging and relatively indolent cancers such as DTC. This study provides preliminary data on construct validity and internal consistent reliability of a tool that may facilitate comparisons in HRQoL domains with other types of cancers or with DTC from other countries.
Concurrent validity was demonstrated for scales of the EORTC QLQ-C30 Tagalog and SF-36 Tagalog measuring similar quality of life dimensions, i.e. physical functioning, social functioning, role functioning, global health, and pain where correlation was moderate. This finding corroborates with other studies which use different translations on populations with a multitude of cancer diagnoses. Correlation coefficients were in the range of 0.40 to 0.62 (physical functional, role functioning, social functioning) in Singapore, 0.34 to 0.53 (global health, social functioning) in Germany and 0.32 to 0.57 (global health, physical, role functioning, social functioning) in Turkey.[17],[18],[19] Weak correlation was found between fatigue and vitality scales in this study as well as emotional functioning and mental health. Reasons for this are unclear. Intuitively, items under fatigue and vitality measure similar concepts relating to loss of energy or tiredness while items for emotional functioning and mental health measure related concepts such as feeling anxious, depressed and happy. Moderate to strong correlations are found in many studies comparing scales of fatigue and vitality, while weak correlation was also found in a study in Turkey and Indonesia comparing emotional and mental functioning.[19]-[20] The weak correlation for the current population may be due to way the respondents interpreted and answered the translated items or the response anchors in one or both questionnaires.
Convergent and discriminant validity was established for the domains of global health, emotional functioning, role functioning, social functioning and nausea and vomiting confirming that the local translation did not compromise the hypothesized structure of the third version of the EORTC QLQ-C30 for these scales. Scaling errors however were noted for the physical functioning domain which had items with low correlation coefficients within its own scale or which correlated more with fatigue (Item 1), or emotional functioning (item 4, 5). One possible explanation for this finding would be that the sampled respondents answered questions on physical function based on how physically tired or worried they were about their illness. These observations were previously reported in studies among Ethiopian and Indonesian cancer patients.[20]-[21] Furthermore, low correlation coefficients for physical functioning items were also reported in Singapore, South Korea and Italy and attributed to skewed responses where the questionnaire was tested on a relatively functional cancer population with few physical impairments similar to the ones enrolled in this study.[17],[22]- [23] For validation studies, sampling a heterogenous population with different disease severity or functional impairment is important as it allows a wide range of responses and yield better correlation.[14]
Items under pain and cognitive functioning also did not meet convergent and discriminant validity criteria and may be due to the effects of translation of some terms. Pain is “sakit” in Tagalog, which is a homonym, and may refer to a sensation, which is the intended construct being measured by the scale in the English version or “sakit” as illness or disease. Some patients may have interpreted questions on pain as the latter thereby resulting in absence of convergence as well as a higher correlation with other functional scales. Item 25 where the term “pag-aalala” was used under cognitive functioning also had a higher correlation with emotional functioning. “Pag-aalala” is another homonym and may mean the act of remembering, the intended concept of item 25, or worry, the concept of item 22 under emotional functioning depending on one’s pronunciation. Like pain, there may have been a variable interpretation by some patients of the meaning of “pag-aalala” thus leading to compromised convergent and discriminant validity.
The global health, role functioning, emotional functioning and nausea and vomiting scales showed good internal consistent reliability with α ranging from 0.77 to 0.88. Save for role functioning, these scales were also the same scales with good psychometric performance when tested on a South Korean population with various cancers.[22] The physical and social functioning, fatigue, pain and cognitive functioning scales had suboptimal reliability with cronbach’s α ranging from 0.38 to 0.64. Low cronbach’s α has been consistently reported for the cognitive functioning scale not only in the English version but also in numerous other translations.[17],[19]-[22],[24],[25],[26] On inspection, items comprising the cognitive functioning scale in the English version already measure distinct aspects of cognition, i.e. memory and concentration, and thus the respective translations in Tagalog also resulted in low inter-relatedness between items. During questionnaire development however, scales with low cronbach’s alpha may be retained if it is outweighed by its clinical relevance in the population it is used on.[14] The suboptimal α for the other scales may be due to the different interpretations by the respondents of constructs especially for scales containing homonymous terms as earlier described. The limitation of this study is that although it enrolled patients at different disease stages and received different types of surgery with none to varying doses of RAI, it still sampled a relatively functional group of patients following up at the outpatient department thus the population may not have been representative enough of the entire DTC population. Although thyroid cancer and its treatment does not usually lead to physical disability compared to other cancers unless there is bone fracture or diffuse lung metastases for instance, it would be more informative to explore how scales perform if more of the respondents included are patients admitted or recently discharged after thyroidectomy, patients immediately post RAI, and patients on chemotherapy or radiotherapy as adding these groups of patients could result in more diverse responses and thus different impairments in the domains and symptom scales of the questionnaire. The study was also done in a single center, introducing selection bias and is another inherent limitation. Moreover, because of the cross-sectional design, the study questionnaire’s test-retest reliability and responsiveness were not tested. Nonetheless, this study provides preliminary evidence supporting the validity and reliability of the EORTC QLQ-C30 Tagalog for certain scales when administered to adult Filipinos with differentiated thyroid cancer.
The EORTC QLQ-C30 Tagalog shows acceptable construct validity and internal consistent reliability for the scales of global health, role functioning, social functioning, emotional functioning, nausea and vomiting when administered to adult Filipinos with differentiated thyroid cancer. Further studies of longitudinal design and more diverse patient populations are recommended to corroborate the study findings and test responsiveness. Improvements in the translation of some items under the pain scale and cognitive functioning may be needed.
AcknowledgmentThe investigators would like to thank Professor Laurie Ramiro of the Department of Behavioral Sciences UP Manila, consultants from the Section of Endocrinology, Diabetes and Metabolism of the UP-Philippine General Hospital and Dr. Venus Oliva Cloma Rosales for their valuable inputs in the construction of the protocol and analysis of data. The authors also recognize Dr. Henry Medina, Isabella Ochoa and Kerry Ong for their assistance in the conduct of the study, encoding and analysis of data.
Statement of AuthorshipAll authors certified fulfillment of ICMJE authorship criteria.
Author DisclosureThe authors declared no conflict of interest.
Funding SourceThis study is supported by a grant from the Philippine Society of Endocrinology, Diabetes and Metabolism (PSEDM).
[1] Husson O, Haak HR., Oranje WA., Mols F., Reemst PH, van de Poll-Franse LV. Health related quality of life among thyroid cancer survivors: A systematic review. Clin Endocrinol (Oxf).2011;75(4):544-54. PubMed CrossRef
[2] Laudico AV, Mirasol-Lumague MR, Medina V, Mapua CA, Valenzuela FG, Pukkala Eero. 2015 Philippine cancer facts and estimates. Manila: Philippine Cancer Society. Retrieved from http://www.philcancer.org.ph/wp-content/uploads/2017/07/2015-PCS-Ca-Facts-Estimates_CAN090516.pdf. Accessed October 2017.
[3] Lo TE, Uy AT, Maningat PD. Well-differentiated thyroid cancer: The Philippine General Hospital experience. Endocrinol Metab (Seoul). 2016;31(1):72-9. PubMed PubMed Central CrossRef
[4] EORTC QLQ-C30 (n.d.) Retrieved from http://groups.eortc.be/qol/eortc-qlq-c30. Accessed October 2016.
[5] Dagmara K, personal communication, February 3, 2017.
[6] Dagmara K, Bottomley A, Velikova G, Greimel E, Koller M on behalf of the EORTC Quality of Life Group. EORTC Quality of Life Group Translation Procedure, 4th edition, 2017.
[7] Zafranco JB, Pangilinan A, Varilla V, Tiangco B. Health Related Quality of Life in Filipino Breast Cancer Patients: Validation of the European Organization for Research and Treatment of Cancer QLQ-C30 and QLQ-BR23. 2009 (Unpublished).
[8] Li RJ, Jimeno C, Sandoval M, Cabungcal A, Ogbac R, Uy G. Development and validation of a thyroid cancer-specific health related quality of life questionnaire for adult Filipinos with differentiated thyroid cancer. J ASEAN Fed Endocr Soc. 2016;31(2):87-96. CrossRef
[9] Bernardo DC on behalf of the Section of Endocrinology, Diabetes and Metabolism. Annual Report Academic Year 2017. University of the Philippines Philippine General Hospital. 2018 January (Unpublished).
[10] Edge SB, Byrd DR, Compton CC, Fritz AG, Green FL, Trotti A. Thyroid cancer staging. In: Edge SB, Byrd DR, Compton CC, Fritz AG, Green FL, Trotti A (eds) AJCC Cancer Staging Manual. 7th edition. New York: Springer; 2010.
[11] Haugen BR, Alexander EK, Bible KC, et al. 2015 American Thyroid Association Management Guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: The American Thyroid Association Guidelines Task Force on thyroid nodules and differentiated thyroid cancer. Thyroid. 2016;26(1):1-133. PubMed PubMed Central CrossRef
[12] Fayers P, Aaronson NK, Bjordal K, Groenvold M, Curran D, Bottomley A. EORTC QLQ-C30 Scoring Manual (3rd edition). 3rd ed. Brussels, Belgium: European Organisation for Research and Treatment of Cancer, 2001.
[13] Castillo-Carandang NT, Sison OT, Grefal ML, et al. A community-based validation study of the short-form 36 version 2 Philippines (Tagalog) in two cities in the Philippines. PLoS ONE. 2013;8(12):e83794. CrossRef
[14] Fayers P, Machin D. Multi-item Scales. In: Quality of life – Assessment, analysis & interpretation. England: John Wiley and Sons, 2000.
[15] Vergara N, Montoya JE, Luna HG, Amapro JR, Cristal-Luna G. Quality of life and nutritional status among cancer patients on chemotherapy. Oman Med J. 2013;28(4):270-4. PubMed PubMed Central CrossRef
[16] Ong A, Andrada P, Dy F, et al. Quality of life among patients with untreated hepatocellular carcinoma. Philipp J Intern Med. 2009;47(3):107-16.
[17] Luo, N, Fones CS, Lim SE, Xie F, Thumboo J, Li SC. The European Organization for Research and Treatment of Cancer Quality of Life Questionnaire (EORTC QLQ-C30): Validation of English version in Singapore. Qual Life Res. 2005;14(4):1181-6. PubMed
[18] Kuenstner S, Langelotz C, Budach V, Possinger K, Krause B, Sezer O. The comparability of quality of life scores. A multitrait multimethod analysis of the EORTCC QLQ-C30, SF-36 and FLIC questionnaires. Eur J Cancer. 2002;38(3):339-48. PubMed
[19] Cankurtaran ES, Ozalp E, Soygur H, Akbiyik DI, Bottomley A. Understanding the reliability and validity of the EORTC QLQ-C30 in Turkish cancer patients. Eur J Cancer Care (Engl). 2008;17(1):98-104. PubMed CrossRef
[20] Perwitasari DA, Atthobari J, Dwiprahasto I, et al. Translation and validation of the EORTC QLQ-C30 into Indonesian version for cancer patients in Indonesia. Jpn J Clin Oncol. 2011;41(4):519-29. PubMed CrossRef
[21] Ayana BA, Negash S, Yusuf L, Tigeneh W, Haile D. Reliability and validity of amharic version of EORTC QLQ-C30 questionnaire among gynecological cancer patients in Ethiopia. PLoS ONE. 2016; 11(6):e0157359. PubMed PubMed Central CrossRef
[22] Lee EH, Chun M, Wang HJ, Lim HY, Choi JH. Multidimensional constructs of the EORTC Quality of Life Questionnaire (QLQ-C30) in Korea cancer patients with heterogenous diagnoses. Cancer Res Treat. 2005;37(3):148-56. PubMed PubMed Central CrossRef
[23] Apolone G, Filiberti A, Cifani S, Ruggiata R, Mosconi P. Evaluation of the EORTC QLQ-C30 questionnaire: A comparison with SF-36 health survey in a cohort of Italian long survival cancer patients. Ann Oncol. 1998;9(5):549-57. PubMed
[24] Aaronson NK, Ahmedzai S, Bergman B, et al. The European Organization for Research and Treatment of Cancer QLQ-C30: A quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst. 1993;85(5):365-76. PubMed
[25] Silpakit C, Sirilerttrakul S, Jirajarus M, Sirisinha T, Sirachainan E, Ratanatharathorn V. The European Organization for Research and Treatment of Cancer Quality of Life Questionnaire (EORTC QLQ-C30): Validation study of the Thai version. Qual Life Res. 2006;15(1):167-72. PubMed CrossRef
[26] Chaukar DA, Das AK, Deshpande MS, et al. Quality of life of head and neck cancer patient: validation of the European Organization for Research and Treatment of Cancer QLQ-C30 and European Organization for Research and Treatment of Cancer QLQ-H&N35 in Indian patients. Indian J Cancer. 2005;42(4):178-84. PubMed
Authors are required to accomplish, sign and submit scanned copies of the JAFES Author Form consisting of: (1) Authorship Certification, that all the requirements for authorship have been met by each author, and that the final version of the manuscript has been read and approved by all authors; (2) the Author Declaration, that the article represents original material that is not being considered for publication or has not been published or accepted for publication elsewhere; (3) the Statement of Copyright Transfer [accepted manuscripts become the permanent property of the JAFES and are licensed with an Attribution-Share Alike-Non-Commercial Creative Commons License. Articles may be shared and adapted for non-commercial purposes as long as they are properly cited]; and the ICMJE form for Disclosure of Potential Conflicts of Interest. For original articles, authors are required to submit a scanned copy of the Ethics Review Approval of their research as well as registration in trial registries as appropriate. For manuscripts reporting data from studies involving animals, authors are required to submit a scanned copy of the Institutional Animal Care and Use Committee approval. For Case Reports or Series, and Images in Endocrinology, consent forms, are required for the publication of information about patients; otherwise, appropriate ethical clearance has been obtained from the institutional review board. Articles and any other material published in the JAFES represent the work of the author(s) and should not be construed to reflect the opinions of the Editors or the Publisher.