Association of Metabolic Syndrome with the Severity of Airflow Obstruction in Patients with Chronic Obstructive Pulmonary Disease*
Gherald Bermudez,* Gabriel Jasul Jr.,* Aileen David-Wang,** Cecilia Jimeno,* Jonray Magallanes,** Anna Angelica Macalalad-Josue*
Gherald R. Bermudez, MD
Section of Endocrinology, Diabetes and Metabolism
Department of Medicine, University of the Philippines-Philippine General Hospital
Taft Avenue, Ermita, Manila, Philippines 1000
Tel. No.: +632-554-8400 local 2230
E-mail: ged_md@yahoo.com
ORCID: https://orcid.org/0000-0002-4776-6885
e-ISSN 2308-118x
Printed in the Philippines
Copyright © 2018 by the JAFES
Received July 6, 2018. Accepted August 8, 2018.
Published Online First: September 26, 2018.
*Presented during the following conventions: 11th Asian Pacific Society of Atherosclerosis and Vascular Diseases (APSAVD), Iloilo Convention Center, Iloilo City, Philippines, Feb. 27 – Mar. 01, 2018 (Oral Presentation); 6th Seoul International Congress of Endocrinology and Metabolism (SICEM), Grand Walkerhill Hotel, Seoul, Korea, April 19 – 22, 2018 (Poster Presentation); 48th Philippine College of Physicians Annual Convention and 5th ASEAN Federation of Internal Medicine Meeting, SMX Convention Center, Manila, Philippines, April 29 – May 2, 2018 (Oral Presentation).
Background. Metabolic Syndrome (MetS) is common in Chronic Obstructive Pulmonary Disease (COPD) patients but their association is still an unsettled issue. The aim of this study was to determine the association of MetS with the severity of airflow obstruction.
Methodology. This was a cross-sectional analytic study of 157 patients with COPD. They were classified using the Global Initiative for Chronic Obstructive Lung Diseases (GOLD). MetS was assessed using two well-recognized criteria. Demographics, clinical data, lifestyle-related characteristics, fasting blood sugar (FBS) and lipid profile were obtained. Multiple logistic regression was used to determine the association of MetS with the severity of airflow obstruction.
Results. 40.13% and 17.20% of patients had MetS using the NCEP/ATP III-AHA/NHBLI and IDF criteria, respectively. MetS was not associated with severity of airflow obstruction. Of the MetS components, only elevated blood pressure (BP) was significantly associated with severity of airflow obstruction (GOLD II: OR=3.28, p<0.001; GOLD III: OR=4.04, p=0.2; GOLD IV: OR=6.21, p=0.04). Elevated FBS was also associated with GOLD IV (OR=16.09, p=0.02). Significant factors associated with MetS in COPD patients were body mass index, inhaled steroid, number of pack-years, and GOLD II.
Conclusion. MetS is not associated with severity of airflow obstruction. Only certain components of MetS showed significant associations such as elevated BP with GOLD II-IV and elevated FBS with GOLD IV.
Keywords: metabolic syndrome, airflow obstruction, chronic obstructive pulmonary diseaseChronic Obstructive Pulmonary Disease (COPD) is the fourth leading cause of death in the world.[1] It coexists with other diseases that may have a significant impact on prognosis. Among these disease entities is the Metabolic Syndrome (MetS), a clustering of metabolic abnormalities that occur in the same individual which appear to confer a substantial additional cardiovascular risk over and above the sum of the risk associated with each abnormality. Studies have shown that the presence of MetS is more frequent in COPD than in those with normal lung function, two common entities that share common inflammatory pathway.[2],[3]
MetS is found to be twice more common in COPD when compared to the general population with prevalence ranging from 25.6 to 60.9%.[4] MetS has been evaluated as a risk factor for chronic lung diseases. The exact nature of the relationship between MetS and lung function impairment remains unknown, and therefore, deserves further investigation.[5]
How people with COPD develop MetS remains unclear, but it has been postulated that its pathogenesis is multifactorial.[6],[7] There are several risk factors such as smoking, genetics, obesity, physical inactivity, and airflow limitation that link the pathogenic mechanism between these two. Among these factors, smoking has the strongest association. The potential mechanism responsible for the development of MetS and COPD in a smoker is primarily due to systemic inflammatory response.[4] Hence, systemic inflammation is considered a hallmark of both MetS and COPD. The existing interaction among the pro-inflammatory proteins released from both pulmonary and adipose tissues and the systemic compartment play a vital role in the development of these two diseases.[6]
Evidences also show the influence of each component risk factor of MetS in patients with COPD. Obesity, the most studied in terms of linking to certain respiratory disorders, decreases expiratory reserve volume and functional residual capacity.[1],[6] Among COPD patients, the prevalence of dyslipidemia and diabetes mellitus was found to be 48.3% and 3-12%, respectively.[4],[8] The latter is even higher in more severe stages of the disease which could be related to the inflammatory process or due to the therapeutic side effect with the use of corticosteroids.[9],[10] In patients with COPD, 31% to 33% are also hypertensive.[11]
In the Philippines, data from the Department of Health show that COPD ranks seventh among the leading causes of mortality.[12] Overall, there are 17.3 million Filipinos who currently smoke tobacco.[13] The prevalence of COPD is higher in men than in women and increases steadily with age from 40 years to more than 70 years.[14]
MetS is also common among Filipinos and low high-density lipoprotein (HDL) is the most common component. The prevalence of MetS in the Philippines is found to be 18.6% by the National Cholesterol Education Program/Adult Treatment Panel III criteria modified by the American Heart Association/National Heart, Lung and Blood Institute (NCEP/ATP III-AHA/NHLBI) criteria. MetS has been shown to be associated with atherosclerotic cardiovascular disease, stroke, and diabetes mellitus.[15]
There is no data that describes the association of MetS with the severity of airflow obstruction among Filipinos with COPD since it is likely that these two entities and their relationship vary from population to population.[16] Because these two diseases are affecting more Filipinos, we need more data for public health intervention.
Among patients with COPD, it is important to identify patients who have MetS early in their course so that early lifestyle intervention and treatment may be initiated. The morbidity[17] and mortality[18] associated with the development of MetS among patients with COPD further intensify the burden of the disease. This study aims to increase awareness among other physicians of the high burden of MetS among COPD patients in our country. This study also hopes to establish the basis for screening MetS among COPD patients. Screening will identify additional MetS cases, facilitating early detection that may help improve COPD treatment outcomes.
The aim of the present study was to determine the association of MetS with the severity of airflow obstruction among patients with COPD in a tertiary government institution, and to determine significant factors associated with MetS in these patients.
METHODOLOGYEligibility criteria
This was a cross-sectional analytic study which included patients aged 40 years old and above diagnosed with COPD using spirometry attending the Pulmonary Medicine outpatient clinic of the University of the Philippines – Philippine General Hospital (UP – PGH), a tertiary government hospital in Manila, Philippines. Patients who were clinically diagnosed with COPD seeking consult for the first time were confirmed by undergoing spirometry. Once eligible, patients were recruited by the investigator to participate in the study.
Those who had an acute exacerbation, i.e., increase in cough, sputum production, worsening dyspnea, or sputum purulence within three weeks,[2] and those with asthma or history of asthma were excluded. In addition, those having any active infectious diseases such as pulmonary tuberculosis either clinically diagnosed or bacteriologically confirmed, inflammatory diseases such as collagen vascular diseases, inflammatory bowel disease that could cause an increase in C-reactive protein (CRP) levels, and those with malignancy were also excluded from the study.
Using NCSS-PASS 12 (Power Analysis and Sample Size) software, the minimum sample size requirement was at least 157 participants based on the logistic regression power analysis of 95%, odds ratio of 1.993, and 5% alpha error.
The study was approved by the University of the Philippines Manila Research Ethics Board and all patients signed an informed consent.
Diagnosis of COPD
The diagnosis of COPD was made according to the Global Initiative for Chronic Obstructive Lung Disease (GOLD) 2015 criteria.[2] A clinical diagnosis of COPD was consistent in any patient who had dyspnea, chronic cough or sputum production, and a history of exposure to risk factors for this disease.
Spirometry was required to make the diagnosis in this clinical context. The presence of a post-bronchodilator FEV1/FVC <0.70 (forced expiratory volume at 1 second/forced vital capacity) confirmed the presence of persistent airflow limitation, thus of COPD.
Severity of airflow obstruction was defined analogously to the GOLD guidelines[2] which are as follows: GOLD I or mild airflow obstruction: FEV1 ≥80 % predicted; GOLD II or moderate airflow obstruction: 50% ≤ FEV1 <80% predicted; GOLD III or severe airflow obstruction: 30% ≤ FEV1 <50% predicted; and GOLD IV or very severe airflow obstruction: FEV1 ≤30 % predicted.
Diagnosis of Metabolic Syndrome
MetS was diagnosed by two well-recognized criteria: the NCEP/ATP III-AHA/NHLBI and the International Diabetes Federation (IDF).
Using the NCEP/ATP-AHA/NHLBI criteria,[19] three out of the five individual risk components fulfilled MetS. They are as follows: elevated waist circumference defined as ≥90 cm in men or ≥80 cm in women; elevated triglycerides defined as ≥1.7 mmol/L or on drug treatment for elevated triglycerides; decreased HDL-C defined as <1.03 mmol/L in men or <1.29 mmol/L in women or on drug treatment for reduced HDL-C; elevated BP defined as >130/85 mmHg or on anti-hypertensive drug treatment in a patient with a history of hypertension; and, elevated FBS defined as ≥5.6 mmol/L or on drug treatment for elevated glucose.
Using the IDF criteria,[20] central obesity defined by waist circumference with ethnicity specific values: ≥90 cm for males and ≥80 cm for females is required for the diagnosis of MetS and two of the four factors previously mentioned. The four factors are the same as the above parameters for elevated triglycerides, decreased HDL-C, elevated FBS except for elevated BP which has higher cut-off value: systolic BP ≥140 mmHg or diastolic BP ≥ 85 mmHg or on treatment of previously diagnosed hypertension. If body mass index (BMI) is >30 kg/m2, central obesity can be assumed and waist circumference does not need to be measured.
Outcome measurements
After recruitment, participants’ medical history, demographic data, and lifestyle-related characteristics were obtained through interviews. On the same visit, participants underwent anthropometric measurements and laboratory testing.
We defined individuals as never smokers (those who never smoked or who smoked fewer than 100 cigarettes in their lifetime), former smokers (those who smoked at least 100 cigarettes in their entire life but were not currently smoking at the time of interview), or current smokers (those who smoked at least 100 cigarettes in their entire life and are still smoking). Pack-years (packs of cigarettes per day multiplied by smoking years) was used as the smoking index.[21] Alcohol intake was likewise probed. Exercise was determined by patient’s self-reporting. Other variables that were collected in the study were COPD-related symptoms, time since diagnosis (in years), and intake of steroids as part of their COPD pharmacotherapy.
Anthropometric measurements such as height in centimeters (cm) and weight in kilograms (kg) were measured after removal of shoes using a wall-mounted stadiometer and a weighing scale, respectively. Waist circumference was measured at the end of normal expiration, midpoint between the top of the iliac crest and the lower margin of the last palpable rib in the mid-axillary line. Hip circumference was measured at the largest circumference of the buttocks.[19],[22] Waist-to-hip circumference ratio was also determined. BMI was calculated as weight in kilograms divided by the height in meters squared (kg/m2). Analyses of BMI was conducted using WHO-defined BMI categories for public health action in Asians: acceptable risk or normal weight (18.5 to <23.0 kg/m2), increased risk or overweight (23.0 to <27.5 kg/m2), and higher high risk or obese (≥27.5 kg/m2).[23] An average BP in mmHg was calculated from two measurements with the subjects in a sitting position after five minutes of rest.
After an overnight fast, five milliliters of venous blood were collected from each patient for measurement of serum levels of total cholesterol, triglycerides, HDL-C, low-density lipoprotein cholesterol (LDL-C) and fasting glucose (FBS). Collected specimens were brought immediately to the laboratory, where they were centrifuged and analyzed.
Patients who were diagnosed with MetS were referred back to their respective attending physicians for counseling, lifestyle intervention, health promotion, and were given necessary treatment based on the current guidelines.
Data analysis
Continuous variables were presented using means and standard deviations (SD) for normally distributed data and median and interquartile range (IQR) for non-normally distributed data, while categorical variables were presented as frequencies and percentages. Normality of distribution for continuous variables was tested using the Shapiro-Wilks test. Student’s T test and Wilcoxon tank sum test were used to compare continuous variables between two groups with normal and non-normal distribution, respectively. Chi-square test was used to compare categorical variables. Prior to variable selection, tests for collinearity, confounding, and interaction were performed. Multicollinearity was assumed if the variable inflation factor was >10. Confounding was considered if the change in odds ratio in the crude and adjusted logistic model was >10%. After checking for interaction, no variables were identified to significantly modify the relationship of COPD and MetS. During the univariate analysis, variables found to be significant at p-value <0.2 were entered into an exploratory multivariate logistic regression model.
After the univariate analysis, variables that were included in the variable selection included age, age at diagnosis of COPD, duration of COPD, use of inhaled steroids, smoking duration, classification of smoking, number of pack years, exercise, BMI, waist-hip ratio, and GOLD classification. Multivariate logistic regression was then performed to examine the factors associated with MetS in patients with COPD. Independent predictors of MetS were identified using the hierarchical method of variable selection. The significant variables (p<0.05) after hierarchical stepwise elimination formed the final predictive model. Statistical analyses were performed using STATA 13.
We recruited 157 participants from August 2017 to April 2018. The demographic, anthropometric, clinical and biochemical, and lifestyle-related characteristics of the study population are summarized in Table 1. Majority of the participants were males (80.89%). Mean (SD) age was 64.3 (9.47) years. Based on the BMI categories for Asians, most of the patients had normal weight. A larger number of patients with COPD were on steroid treatment (68.15%), previous smokers (80.89%), and alcohol drinkers (82.17%). The median number of pack-years was 34 years while the median number of smoking duration was 30 years. Mean forced expiratory volume at 1 second (FEV1) of the study patients was 0.78. Most subjects recruited were classified as COPD GOLD I (45.22%). 36 (23%) had elevated waist circumference, 30 (19%) had elevated triglycerides, 57 (36%) had reduced HDL-C, 72 (46%) were hypertensive, and 62 (39%) had elevated FBS.
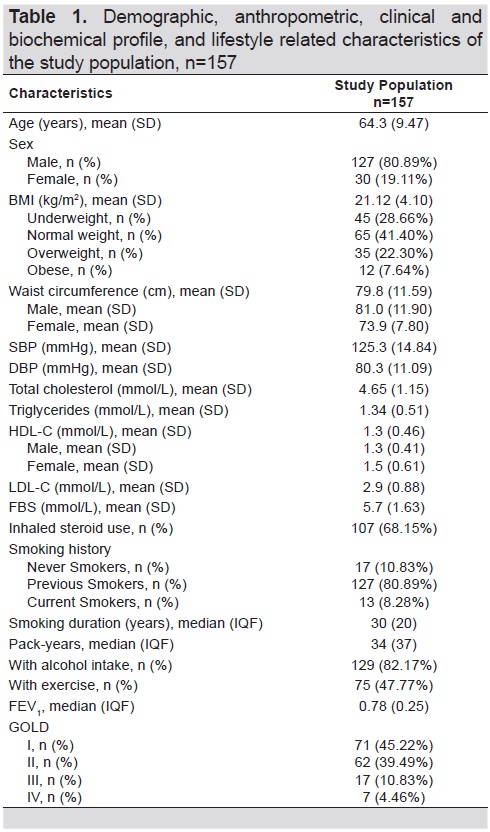
Table 1. Demographic, anthropometric, clinical and biochemical profile, and lifestyle related characteristics of the study population, n=157
Table 2 shows the differences in the characteristics of patients with and without MetS. Significant characteristics of patients associated with MetS were seen in relation to smoking history, smoking duration, steroid use, exercise and number of pack-years. Most patients with MetS were previous smokers. Of those with MetS, only six patients were still smoking at the time of recruitment while two were never smokers. Majority of patients who were never smokers were females. Most of them had occupational-related COPD such as concrete-manufacturing workers, street vendors and coal-miners while others were second-hand smokers. Among those who were on steroid treatment, all were on maintenance inhaled steroids.

Table 2. Comparison of demographic and clinical characteristics of patients with and without MetS
Table 3 shows the prevalence of MetS according to various criteria with the severity of airflow limitation in patients with COPD. Both the NCEP-ATP III-AHA/NHLBI and the IDF criteria were good criteria in diagnosing MetS across GOLD classification in patients with COPD. However, a larger number of patients was diagnosed with MetS using the NCEP/ATP III-AHA/NHLBI criteria [n=63 (40.13%)] in comparison to the IDF criteria [n=27 (17.20%)]. In the GOLD classification, GOLD II has the largest percentage of patients with MetS using both criteria (52.38% in the NCEP/ATP III-AHA/NHLBI while 51.85% in the IDF criteria).
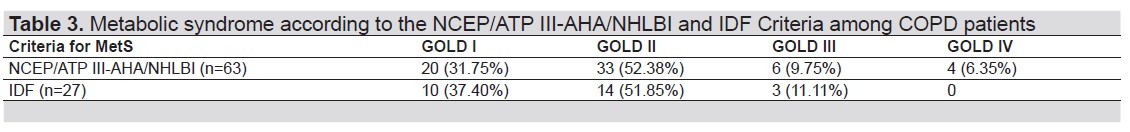
Table 3. Metabolic syndrome according to the NCEP/ATP III-AHA/NHLBI and IDF criteria among COPD patients
Among the individual risk components of MetS as shown in the multivariate analysis in Table 4, only elevated BP was significantly and consistently associated with the severity of airflow obstruction (OR=3.28, 95% CI 1.59-6.76, p<0.001 in GOLD II; OR=4.04, 95% Cl 1.28-12.69, p=0.2 in GOLD III; OR=6.21, 95% CI 1.07-36.10, p=0.04 in GOLD IV). In addition, elevated FBS was significantly associated with GOLD IV (OR=16.09, 95% CI 1.44-179.50, p=0.02).
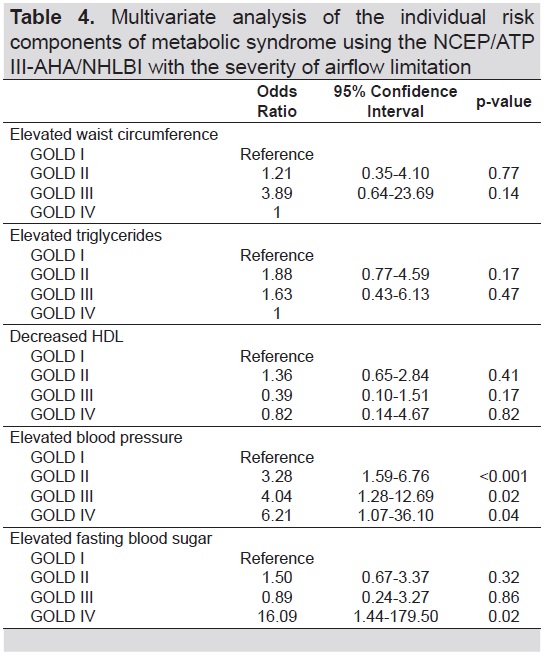
Table 4. Multivariate analysis of the individual risk components of metabolic syndrome using the NCEP/ATP III-AHA/NHLBI with the severity of airflow limitation
In the multivariate logistic analysis shown in Table 5, MetS was not associated with increasing severity of airflow obstruction in COPD patients. However, other factors significantly associated with MetS in patients with COPD were inhaled steroid use, BMI, number of pack-years, and GOLD II. For every 1 unit increase in BMI, there is a 31% increase in the odds of having MetS. Similarly, for every 1 additional pack year incurred, there is a 3% increase in the odds of having MetS. Those who used inhaled steroid as part of their COPD pharmacotherapy have threefold chance of having MetS. Also, among the GOLD classification, patients classified as GOLD II were three times more likely to have MetS.
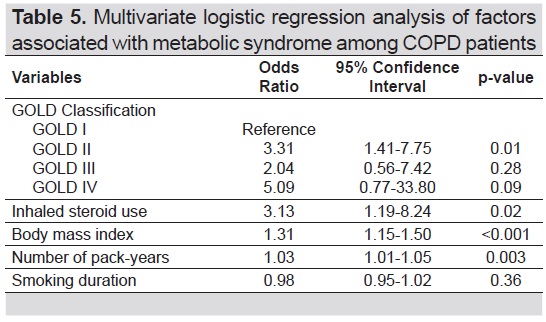
Table 5. Multivariate logistic regression analysis of factors associated with Metabolic Syndrome among COPD patients
The results of our study showed that MetS was not associated with the severity of airflow obstruction. However, we found that certain components of MetS were associated with the severity of airflow obstruction and that the prevalence of MetS in COPD was higher than in the general population.[4] Among the individual risk components of MetS, elevated BP was shown to be significantly associated with airflow obstruction across all GOLD classification. On the other hand, elevated FBS was significantly associated with very severe airflow obstruction or GOLD IV. Also, the rate of MetS among patients with COPD in the present study was 40.13% using the NCEP/ATP III-AHA/NHLBI.
Two previous reports showed that MetS was associated with impaired lung function but this relationship was only significant with restrictive lung impairment and not airflow obstruction.[24],[25] However, in the present analysis, certain components of MetS were significantly associated with airflow obstruction. We found out that among these components, increased BP was the main effect linked with airflow obstruction. This resonates with reports of previous studies in various settings. Marquis et al.,[26] reported that among MetS components, high BP, in addition to abdominal obesity, were more frequent in COPD men. Recent analysis of data from 20,296 subjects which included 11,258 men and 9,038 women aged ≥;45 years in two large combined cohort studies showed that subjects with GOLD stage III or IV had a higher prevalence of hypertension.[3] The INDACO project in Italy, a pilot study on the incidence of comorbidities in COPD patients referred to pneumology units, showed a 52.1% prevalence rate of hypertension among COPD patients.11 Watz et al.,[27] in their study, also showed that hypertension was highly prevalent across all groups of COPD patients. A recent systematic review even showed that the most prevalent component of MetS in COPD was hypertension.[28] The pathological mechanisms responsible for hypertension in COPD are hypoxia-related vasoconstriction, free radical injury, endothelial dysfunction, and arterial stiffness.[4] Control of hypertension in COPD subjects can decrease cardiovascular-related mortality.[29] Note that these were observational studies comparing severity of airflow obstruction with normal lung function. We found no studies comparing severity of airflow obstruction using GOLD 1 as reference standard.
In contrast with our results, Lam et al.,[30] detected no association between airflow obstruction and increased BP. They found that among the five components of MetS, only central obesity was significantly associated with airflow obstruction.
We also found out that increased FBS was associated with very severe airflow obstruction. The prevalence of hyperglycemia in COPD is approximately about 3-12%. Systemic inflammation and steroid use could be important contributory factors responsible for both COPD and hyperglycemia.[4] Similarly, Mannino et al.,[3] also found out that COPD patients with severe and very severe airflow obstruction had a higher prevalence of diabetes (OR 1.5, 95% CI 1.1–1.9).
Across several studies on various countries, the frequency of MetS was highest in COPD GOLD II.[27],[31],[32] This could be due to a relatively higher influence of lifestyle on the body composition and metabolic health in the earlier stages of COPD in comparison to the COPD-induced triggers on wasting process in the more severe stages.[28] Moreover, in most of these studies, the most number of patients recruited were classified as GOLD II. This could also explain the higher prevalence of MetS in those with early stages of COPD. This was also true with our results that the frequency of MetS was significantly highest in COPD GOLD II. This was supported by the previous studies mentioned earlier.
Indeed, the prevalence of MetS in COPD is highly variable among studies. This is dependent on the criteria being used to diagnose MetS, the study inclusion criteria and the country or ethnicity being studied.[32] Several pathogenic mechanisms have been proposed which tried to establish the link between MetS and COPD, however, these are still poorly understood. These mechanisms include common pathophysiological mechanisms such as systemic inflammation, adipose tissue inflammation, physical inactivity, hypogonadism and the effect of steroids.[6],[33],[34]
It is notable that MetS was significantly higher using the NCEP/ATP-AHA/NHLBI criteria in comparison with IDF. This is consistent with the study of Morales et al.,[15] that among the different criteria of MetS, the NCEP/ATP-AHA/NHLBI criteria was able to detect most number of MetS compared with other diagnostic criteria. Moreover, the present NCEP/ATP-AHA/NHLBI statement, in contrast to IDF, maintains the previous ATP III criteria established in 2001 except that the threshold for impaired fasting glucose was reduced from 110 mg/dl to 100 mg/dl, which corresponds to the criteria used by the American Diabetes Association which was eventually adapted in our local setting. In contrast, the IDF clinical definition for MetS makes the presence of abdominal obesity necessary for its diagnosis, which could be one reason that MetS is lower in Filipinos using the IDF criteria.[19]
Smoking is an established risk factor for COPD and has been associated with increased MetS prevalence and increased cardiovascular disease risk.[35] Given that this has been suggested as one of the major causes of systemic inflammation in association with COPD and MetS, the higher frequency of smokers in our sample may have contributed to the higher frequency of MetS. This is further supported by a population-based survey conducted in nine Asia-Pacific territories which revealed that among Filipinos with COPD, more than half were smokers.[36] Current statistics also indicate that nearly 30% of adult Filipinos smoke making COPD the seventh cause of mortality in our local setting.[37] The potential mechanism responsible for development of COPD and the MetS in a smoker is primarily due to systemic inflammatory response.[4]
Lastly, among the factors associated with MetS and COPD, we found out that BMI, number of pack-years, inhaled steroid use, and GOLD II were significant factors associated with MetS in COPD patients. Steuten et al.,[38] conducted a study to look at the association of severity of COPD and BMI and found out that COPD severity is affected by increasing BMI. A recently published study from Italy showed a statistical correlation among pack-years and the development of MetS.[39] There is a positive dose–response relationship between the daily number of cigarettes smoked and the duration of smoking and the risk of MetS. Even former smokers are at increased risk for the development of Mets because the risk has been shown to even persist for up to 20 years after smoking cessation.[40],[41]Additionally, MetS is substantial in early stages of COPD such as GOLD I and GOLD II.[42]
The metabolic effects of systemic glucocorticoid therapy are well known affecting various parameters of the MetS.[4] In comparison with the oral or parenteral routes, inhaled corticosteroids (ICS) are known to produce relatively less systemic adverse effects.[43] All participants in our study who were on steroid therapy were using ICS. Our findings showed that those who were treated with ICS were three times more likely to have MetS. Though the systemic bioavailability of ICS is claimed to be minimal, a significant proportion of ICS can still reach the systemic circulation especially at high doses. In addition, the effects of ICS on the circulation is also dependent on the daily dosage as well as its pharmacodynamic and pharmacokinetic properties.[43] This could potentially explain the relationship of ICS and MetS in our study as we did not explore further the dosages of the ICS our study participants were taking.
The current analysis has few limitations. First, this study was a cross-sectional study which made it difficult to adequately describe the causal relationships of detected associations. Second, since systemic inflammatory response is a potential mechanism linking Mets and COPD, this study did not measure any marker of inflammation such as CRP, fibrinogen, interleukin-6, etc. Prospective longitudinal studies are needed for better understanding of the temporal relationship between MetS components and systemic inflammatory profile in patients with COPD among Filipinos. Third, we did not gather data on dietary intake as a possible confounder or effect modifier of the association of MetS and severity of airflow obstruction in patients with COPD. Lastly, we were not able to explore the dosages of ICS used by the participants as this may ascertain the potential association of ICS treatment and MetS in our study.
In conclusion, our results showed that MetS was not associated with the severity of airflow obstruction. However, certain components of MetS such as elevated BP and elevated FBS were associated with the severity of airflow obstruction with the latter linked to very severe airflow obstruction or GOLD IV. Factors that were significantly associated with MetS among patients with COPD were BMI, use of inhaled steroids, and number of pack-years. COPD patients with moderate airflow obstruction were also three times more likely to have MetS. We recommend screening for MetS with early stages of COPD.
AcknowledgmentThe authors would like to thank the Endocrinology and Pulmonary Medicine Fellows-in-Training of the UP – PGH for their invaluable support of this project; the Committee on Lipid Research Award of the Philippine Lipid and Atherosclerosis Society (PLAS); the staff of the Medicine Research Laboratory (MRL) of UP - PGH; and, our statisticians.
Statement of AuthorshipAll authors certified fulfillment of ICMJE authorship criteria.
Author DisclosureThe authors declared no conflict of interest.
Funding SourceThis study was funded by a research grant awarded by the Philippine Lipid and Atherosclerosis Society and Pfizer, Inc. (PLAS-Pfizer).
[1] Mirrakhimov AE. Chronic obstructive pulmonary disease and glucose metabolism: A bitter sweet symphony. Cardiovasc Diabetol. 2012;11(1):132. PubMed PubMed Central CrossRef
[2] Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease (updated 2015), 2015. http://www.goldcopd.org/
[3] Mannino DM, Thorn D, Swensen A, Holguin F. Prevalence and outcomes of diabetes, hypertension and cardiovascular disease in COPD. 2008;32(4):962-9. PubMed CrossRef
[4] Naik D, Joshi A, Paul TV, Thomas N. Chronic obstructive pulmonary disease and the metabolic syndrome: Consequences of a dual threat. Indian J Endocrinol Metab. 2014;18(5):608-16. PubMed PubMed Central CrossRef
[5] Baffi CW, Wood L, Winnica D, et al. Metabolic syndrome and the lung. Chest. 2016;149(6):1525-34. PubMed PubMed Central CrossRef
[6] Clini E, Crisafulli E, Radaeli A, Malerba M. COPD and the metabolic syndrome: An intriguing association. Intern Emerg Med. 2013;8(4):283-9. PubMed CrossRef
[7] Park SK, Larson JL. The relationship between physical activity and metabolic syndrome in people with chronic obstructive pulmonary disease. J Cardiovasc Nurs. 2014;29(6):499-507. PubMed PubMed Central CrossRef
[8] de Lucas-Ramos P, Izquierdo-Alonso JL, Rodriguez-Gonzalez Moro JM, et al. Chronic Obstructive pulmonary disease as a cardiovascular risk factor. (CONSISTE Study). Int J Chron Obstruct Pulmon Dis. 2012:679-86. PubMed PubMed Central CrossRef
[9] Gläser S, Krüger S, Merkel M, Bramlage P, Herth FJ. Chronic obstructive pulmonary disease and diabetes mellitus: A systematic review of the literature. Respiration. 2015;89(3):253-64. PubMed CrossRef
[10] Lee CT, Mao IC, Lin CH, Lin SH, Hsieh MC. Chronic obstructive pulmonary disease: A risk factor for type 2 diabetes: A nationwide population-based study. Eur J Clin Invest. 2013;43(11):1113-9. PubMed CrossRef
[11] Fumagalli G, Fabiani F, Forte S, et al. INDACO Project : A pilot study on incidence of comorbidities in copd patients referred to pneumology units. 2013:8(1):28. PubMed PubMed Central CrossRef
[12] Department of Health. The 2013 Philippine Health Statistics. Manila, Philippines, 2013. https://www.doh.gov.ph/sites/default/files/publications/2013PHScompressed_0.pdf
[13] 2009 Philippines’ Global Adult Tobacco Survey Country Report. Manila, Philippines, 2010. http://www.who.int/tobacco/surveillance/2009_gats_report_philippines.pdf
[14] Idolor LF, De Guia TS, Francisco NA, et al. Burden of obstructive lung disease in a rural setting in the Philippines. 2011;16(7):1111-8. PubMed CrossRef
[15] Morales DD, Punzalan FE, Paz-Pacheco E, Sy RG, Duante CA, National Nutrition and Health Survey, 2003 Group. Metabolic syndrome in the Philippine general population: Prevalence and risk for atherosclerotic cardiovascular disease and diabetes mellitus. Diab Vasc Dis Res. 2008;5(1):36-43. PubMed CrossRef
[16] Acharyya A, Shahjahan MD, Mesbah FB, Dey SK, Ali L. Association of Metabolic syndrome with chronic obstructive pulmonary disease in an Indian population. Lung India. 2016;33(4):385-90. http://www.lungindia.com/text.asp?2016/33/4/385/184871
[17] Díez-Manglano J, Barquero-Romero J, Almagro P, et al. COPD Patients with and without Metabolic Syndrome: Clinical and functional differences. Intern Emerg Med. 2014;9(4):419-25. PubMed CrossRef
[18] Tanni SE, Zamuner AT, Coelho LS, Vale SA, Godoy I, Paiva SA. Are metabolic syndrome and its components associated with 5-year mortality in chronic obstructive pulmonary disease patients ? Metab Syndr Relat Disord. 2015;13(1):52-4. PubMed CrossRef
[19] Grundy SM, Cleeman JI, Daniels SR, et al. Diagnosis and management of the metabolic syndrome: An American Heart Association/National heart, Lung, and Blood Institute scientific statement. Circulation. 2005;112(17):2735-52. PubMed CrossRef
[20] Alberti G, Zimmet P, Shaw J, Grundy S. The IDF Consensus Worldwide Definition of the Metabolic Syndrome. Brussels, Belgium, 2006.
[21] Schoenbom CA, Adams PE. Health Behaviors of adults: United States, 2005-2007. Vital Health Stat 10. 2010;(245):132. PubMed
[22] World Health Organization. Waist circumference and waist-hip ratio. Report of a WHO expert consultation, Geneva, 8-11 December 2008. http://apps.who.int/iris/bitstream/handle/10665/44583/9789241501491_eng.pdf?ua=1
[23] WHO Expert Consultation. Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet. 2004;363(9403):157-63. PubMed CrossRef
[24] Fimognari FL, Pasqualetti P, Moro L, et al. The Association between metabolic syndrome and restrictive ventilatory dysfunction in older persons. J Gerontol A Biol Sci . 2007;62(7):760-5. PubMed
[25] Lin WY, Yao CA, Wang HC, Huang KC. Impaired lung function is associated with obesity and metabolic syndrome in adults. Obesity (Silver Spring). 2006;14(9):1654-61. PubMed CrossRef
[26] Marquis K, Maltais F, Duguay V, et al. The metabolic syndrome in patients with chronic obstructive pulmonary disease. J Cardiopulm Rehabil. 2005;25(4):226-32. PubMed
[27] Watz H, Waschki B, Kirsten A, et al. The metabolic syndrome in patients with chronic bronchitis and COPD: Frequency and associated consequences for systemic inflammation and physical inactivity. Chest. 2009:136(4):1039-46. PubMed CrossRef
[28] Cebron Lipovec N, Beijers RJ, can den Borst B, Doehner W, Lainscak M, Schols AM. The prevalence of metabolic syndrome in chronic obstructive pulmonary disease: A systematic review. COPD. 2016;13(3):399-406. PubMed CrossRef
[29] Dart RA, Gollub S, Lazar J, Nair C, Schroeder D, Woolf SH. Treatment of systemic hypertension in patients with pulmonary disease: COPD and asthma. Chest. 2003;123(1):222-243. PubMed
[30] Lam KB, Jordan RE, Jiang CQ, et al. Airflow obstruction and metabolic syndrome: The Guangzhou biobank cohort study. Eur Respir J. 2010;35(2):317-23. PubMed CrossRef
[31] Akpınar EE, Akpınar S, Ertek S, Sayın E, Gülhan M. Systemic inflammation and metabolic syndrome in stable COPD patients. Tuberk Toraks. 2012;60(3):230-7. PubMed
[32] Vujic T, L Nagorni O, Maric G, Popovic L, Jankovic J. Metabolic syndrome in patients with chronic obstructive pulmonary disease: Frequency and relationship with systemic inflammation. Hippokratia. 2016:20(2):110-4. PubMed PubMed Central
[33] Magnussen H, Watz H. Systemic inflammation in chronic obstructive pulmonary disease and asthma: Relation with comorbidities. Proc Am Thorac Soc. 2009;6(8):648-51. PubMed CrossRef
[34] Barnes PJ, Celli BR. Systemic manifestations and comorbidities. Eur Respir J. 2009;33(5):1165-85. PubMed CrossRef
[35] Sun K, Liu J, Ning G. Active smoking and risk of metabolic syndrome: A meta-analysis of prospective studies. PLoS One. 2012;7(10):e47791. PubMed PubMed Central CrossRef
[36] Lim S, Lam DC, Muttalif AR, et al. Impact of chronic obstructive pulmonary disease (COPD) in the Asia-Pacific region: The EPIC Asia population-based survey. Asia Pac Fam Med. 2015:14(1):4. PubMed PubMed Central CrossRef
[37] COPD prevalence in Phl on ‘ high side.’ Philippine Star Health and Medicine, 2015. http://www.pchrd.dost.gov.ph/index.php/news/library-health-news/4326-copd-prevalence-in-phl-on-high-side Accessed September 20, 2016.
[38] Steuten LMG, Creutzberg EC, Vrijhoef HJM, Wouters EF. COPD as a multicomponent disease : Inventory of dyspnoea , underweight , obesity and fat free mass depletion in primary care. Prim Care Respir J. 2006:15(2):84-91. PubMed CrossRef
[39] Cena H, Tesone A, Niniano R, Cerveri I, Roggi C, Turconi G. Prevalence rate of metabolic syndrome in a group of light and heavy smokers. Diabetol Metab Syndr. 2013:5:28. PubMed PubMed Central CrossRef
[40] Balhara YPS. Tobacco and metabolic syndrome. Indian J Endocrinol Metab. 2012;16(1):81-7. PubMed PubMed Central CrossRef
[41] Piazzolla G, Castrovilli A, Liotino V, et al. Metabolic syndrome and chronic obstructive pulmonary disease (COPD): The interplay among smoking , insulin resistance and vitamin D. PloS One. 2017;12(10): e0186708. PubMed PubMed Central CrossRef
[42] Ghatas T. The relationship between metabolic syndrome and chronic obstructive pulmonary disease. Egypt J Bronchol. 2017;11(1)11-5. CrossRef
[43] Egbuonu F, Antonio FA, Edavalath M. Effect of inhaled corticosteroids on glycemic status. Open Respir Med J. 2014;8:101-5. PubMed PubMed Central CrossRef
Authors are required to accomplish, sign and submit scanned copies of the JAFES Author Form consisting of: (1) Authorship Certification, that all the requirements for authorship have been met by each author, and that the final version of the manuscript has been read and approved by all authors; (2) the Author Declaration, that the article represents original material that is not being considered for publication or has not been published or accepted for publication elsewhere; (3) the Statement of Copyright Transfer [accepted manuscripts become the permanent property of the JAFES and are licensed with an Attribution-Share Alike-Non-Commercial Creative Commons License. Articles may be shared and adapted for non-commercial purposes as long as they are properly cited]; and the ICMJE form for Disclosure of Potential Conflicts of Interest. For original articles, authors are required to submit a scanned copy of the Ethics Review Approval of their research as well as registration in trial registries as appropriate. For manuscripts reporting data from studies involving animals, authors are required to submit a scanned copy of the Institutional Animal Care and Use Committee approval. For Case Reports or Series, and Images in Endocrinology, consent forms, are required for the publication of information about patients; otherwise, appropriate ethical clearance has been obtained from the institutional review board. Articles and any other material published in the JAFES represent the work of the author(s) and should not be construed to reflect the opinions of the Editors or the Publisher.