Assoc. Prof. Somsak Tiamkao, MD
Division of Neurology, Department of Medicine,
Faculty of Medicine, Khon Kaen University, Khon Kaen, Thailand
Tel. No.: 66-43-363-225
Fax No.: 66-43-347-542
E-mail: somtia@kku.ac.th
ORCiD: https://orcid.org/0000-0001-5178-478X
e-ISSN 2308-118x
Printed in the Philippines
Copyright © 2019 by the JAFES
Received February 26, 2019. Accepted June 16, 2019.
Published Online First: October 28, 2019.
Introduction. Both myasthenia gravis (MG) and autoimmune thyroid diseases (AITDs) are autoimmune diseases. Graves’disease (GD) is the most common AITD reported to be associated with MG. Currently, there is limited data on prevalence and clinical features/outcomes of MG in various thyroid diseases in a large database report.
Methodology. A total of 872 patients with MG and 97,251 patients with thyroid disorders had been recorded by the tertiary hospital database. The study period was between 1997 and 2017. Patients with a thyroid disorder and MG were identified by the ICD-10-CM code. Clinical courses of MG accompanied by thyroid disorders were studied.
Results. During the 20-year study period, there were 872 patients with MG and 97,251 patients with thyroid disorders. In the group with thyroid disorders, 28,886 patients (29.70%) had GD, 1,612 patients (1.66%) had Hashimoto’s thyroiditis, 13,172 patients (13.54%) had toxic goiter and 53,581 patients (55.10%) had nontoxic goiter. 97 patients had been diagnosed with both MG and thyroid disorders. Among the four types of thyroid disorders, the rate of MG was highest in HT group (9.92/1,000 HT patients). There were four significant factors among four groups of thyroid disorders including age of onset of thyroid disease (p 0.004), MG classification (p<0.001), MG treatment (p<0.001), and thymic pathology (p 0.034). Among the four groups of thyroid disorders, patients with MG and HT were diagnosed with thyroid disease at the youngest age (27 years) compared with other thyroid diseases. Additionally, the MG patients with HT also had the highest proportion of MG class 4-5 a/b (7 patients, 43.75%), received prednisolone treatment (15 patients, 93.75%), received immunosuppressants (9 patients, 56.25%), received IVIG or PLEX (5 patients, 31.30%), and had thymoma (6 patients, 46.15%).
Conclusion. MG is most prevalent in patients with HT. Patients with both MG and HT had more severe MG status and had higher rate of thymoma.
Keywords: autoimmune thyroid diseases, prevalence, treatmentMyasthenia gravis (MG) is an autoimmune disease that results from the binding of autoantibodies to proteins involved in signaling at the neuromuscular junction (NMJ) causing the failure of neuromuscular transmission. These proteins are called the nicotinic acetylcholine receptors (AChR) or, less frequently, a muscle-specific tyrosine kinase (MuSK) receptor involved in AChR clustering. Much is known about the mechanisms that maintain self-tolerance and modulate anti-AChR Ab synthesis, AChR clustering, and AChR function. As a result, nerve impulses cannot trigger muscle contractions.[1],[2] The hallmark of myasthenia gravis is muscle weakness that worsens after use of affected muscles and improves after periods of rest. About two-thirds of patients present with extrinsic ocular muscle weakness that usually progresses to other muscles, resulting in generalized MG. In about 10%, however, symptoms remain limited to the ocular muscle and this condition is termed ocular MG.[3]
The incidence of MG is about 1 to 2 per 100,000 each year while the prevalence is estimated to be as high as 20 to over 50 per 100,000 in the population.4 The incidence of MG is increasing over time due to either improvements in diagnosis or modern treatments so patients live longer with the disease.>[4] The distribution is affected by both gender and age in a bimodal fashion. It is more prevalent in women than men in the second and third decades, while in the sixth and seventh decades it affects more men. It is rare in children less than ten years of age.[5] The idea that MG is an autoimmune disease has been applied to other autoimmune disorders of the neuromuscular junction.[6] Patients with MG may have coexisting autoimmune thyroid diseases (AITDs) as well as other autoimmune disorders such as type 1 diabetes mellitus, primary hypogonadism, pernicious anemia, and adrenal insufficiency, known as the polyglandular syndrome.
The thyroid gland is essential for normal human development and maintenance. Rennie G. described the coexistence of Graves’ disease (GD) with MG for the first time in 1908.[7] These coexisting diseases have been reported more frequently afterward.[8] Although the pathogenic link between these two autoimmune diseases remains unclear, an immunological cross-reactivity between the neuromuscular junction and thyroid components was found in overlapping GD and MG.[9] A report found that various thyroid disorders can be seen with MG including hyperthyroidism, hypothyroidism, nontoxic goiter, Hashimoto’s thyroiditis and the thyroid antibody-positive euthyroid state.[10] Epidemiological studies showed that thyroid disorders occur in approximately 5–10% of MG patients,[11],[12] a fairly low incidence of MG (0.2 %) has been reported in patients with GD. Currently, there are limited data on prevalence and clinical features/outcomes of MG in various thyroid diseases in a large database report.
Population
The data here were used from the ICD-10 diagnostic coding system at Srinagarind Hospital, which is a referral university hospital for the Northeast of Thailand from June 1, 1997 to June 1, 2017. Patients with MG were identified by code G700. Thyroid disorders were divided into diffuse toxic goiter (GD) (code E05.0), toxic nodular/multinodular goiter (codes E05.10 E05.11 E05.20 E05.21), Hashimoto’s thyroiditis (HT) (code E06.3), non-functional thyroid nodule/goiter which included simple goiter, nontoxic nodular/multinodular goiter and thyroid cancer (codes E04.9 E04.0; E04.1 E04.2, C73). The inclusion criteria were adult patients with age of 18 years or over and had diagnosis of MG with any thyroid disorders mentioned earlier.
Medical records of eligible patients were reviewed. The studied variables included gender, age, geographic area, employment status, comorbid diseases, age at diagnosis of thyroid disease/MG, clinical course, treatments and thymic pathology. Both neurological and thyroid evaluation in eligible patients were based on data obtained from medical records as follows:
Neurological evaluation
MG was diagnosed by patient clinical characteristics, pharmacological, serological, and electrodiagnostic data. The diagnosis was confirmed by amelioration of muscle weakness during chronic treatment with pyridostigmine. A mediastinal CT or MRI was performed and patients with thymic abnormalities underwent thymectomy. The diagnosis of thymic hyperplasia or thymoma was based on histological findings. The severity of MG was classified using the Osserman criteria and was divided into the following groups:
- Class 1: Ocular MG (OMG)
- Class 2A: Mild generalized MG (GMG) with no bulbar involvement; Class 2B: mild GMG with bulbar involvement
- Class 3A: Moderate GMG with no bulbar involvement; Class 3B: moderate GMG with bulbar involvement
- Class 4A: Severe GMG with no bulbar involvement; Class 4B: severe GMG with bulbar involvement
- Class 5:Defined by intubation with or without mechanical ventilation, except when employed during routine postoperative management
The patients were categorized as OMG when the symptoms were restricted to the ocular system for two years or more. Treatment of MG included acetylcholinesterase inhibitors (AChEI), immunosuppressants such as corticosteroids, azathioprine, methotrexate, as well as mycophenolate mofetil (MMF), intravenous immunoglobulin (IVIG) and plasma exchange (PLEX).
Thyroid evaluation
Thyroid dysfunction was evaluated, which included physical examinations, thyroid ultrasonography, and thyroid function tests; free thyroid hormones (free T4 and free T3), TSH. The following tests were also carried out when necessary: anti-thyroglobulin autoantibodies (TgAb), anti-thyroid peroxidase autoantibodies (TPOAb), anti-TSH receptor autoantibodies (TRAb), thyroid scans, and thyroid fine needle aspirations. Thyroid diseases were categorized into four groups as follows: GD, HT, toxic goiter, and non-toxic goiter. Diagnosis of thyroid diseases in this cohort was classified based on primary diagnosis of thyroid diseases prior to thyroidectomy or I-131 therapy.
The diagnosis of GD was based on the presence of hyperthyroidism and/or Graves’ ophthalmopathy associated with diffuse goiters and circulating TRAb. All patients with primary hypothyroidism associated with positive TgAb/TPOAb and patients with positive TgAb/TPOAb associated with a firm goiter and a hypoechogenic pattern on ultrasound examination of the gland and/or had lymphocytic infiltration on fine needle aspiration were considered to have HT. Toxic nodular/multinodular goiters have a spectrum of different clinical entities, ranging from a single hyperfunctioning nodule within an enlarged thyroid gland, to multiple hyperfunctioning areas scattered throughout the gland barely distinguishable from nonfunctioning nodules and extranodular parenchyma. Nonfunctioning thyroid nodules or non-toxic goiters were diagnosed in patients with a nodule or goiter associated with normal levels of thyroid hormones including thyroid cancer.
Statistical analysis
The frequency rates per 1,000 population of MG in each thyroid disorder were calculated using incident cases of MG with thyroid disorders as the numerator and incident case of each thyroid disorder as the denominator and frequency rate of thyroid disorders in MG was calculated using the same numerator but the incident case of MG as the denominator. Confidence interval (CI) estimates were based on the Poisson distribution.
MG-related clinical factors were studied and categorized by various types of thyroid disorders. Data were presented as numbers (percentage) or mean (SD) in each type of thyroid disorders. Among the four types of thyroid disorders, differences of studied variables were compared by Fisher Exact test or Krukal-Wallis test for proportions or numerical variables, respectively. Both statistical tests were used due to small sample size or non-normally distributed data for comparing more than two groups. Statistical analyses were performed by the SPSS software package, version 15.0 (SPSS Inc., Chicago, IL, USA) for Windows.
During the 20-year study period, there were 872 MG patients and 97,251 patients with thyroid disorders. Female sex was predominant in both diseases (613/872 or 70.30% in MG and 76,840/97,251 or 79.01% in thyroid disorders). In the group with thyroid disorders, 28,886 patients (29.70%) had GD, 1,612 patients (1.66%) had HT, 13,172 patients (13.54%) had toxic goiter and 53,581 patients (55.10%) had nontoxic goiters. 97 patients had both MG and thyroid disorders; 86 patients (88.66%) were female giving a female: male ratio of 7.8:1. Among the four types of thyroid disorders, the highest number of patient with MG was found in GD (52/97 or 53.61%), but the rate of MG in each thyroid disorder was highest in HT group (9.92/1,000 HT patients) as shown in Table 1.
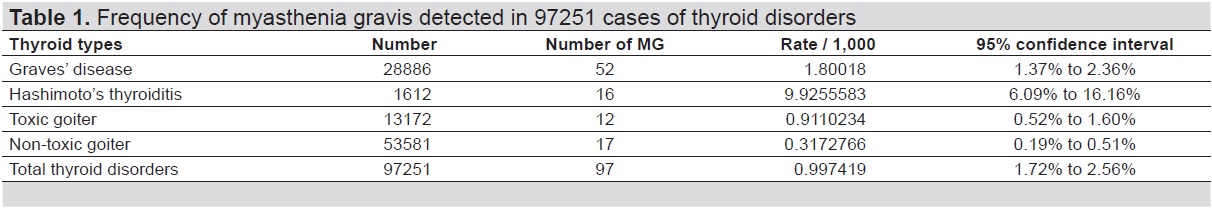
Table 1. Frequency of myasthenia gravis detected in 97,251 cases of thyroid disorders
Clinical characteristics
Table 2 summarizes MG clinical characteristics by various thyroid disorders. There were four significant factors among four groups of thyroid disorders including age of onset of thyroid disease (p 0.004), MG classification (p<0.001), MG treatment (p<0.001), and thymic pathology (p 0.034). Among the four groups of thyroid disorders, MG patients with HT had been diagnosed with thyroid disease at the youngest age (27 years) compared with other thyroid diseases. Additionally, the MG patients with HT also had highest proportions of MG class 4-5 a/b (7 patients, 43.75%), received prednisolone treatment (15 patients, 93.75%), received immunosuppressants (9 patients, 56.25%), received IVIG or PLEX (5 patients, 31.30%), and had thymoma (6 patients, 46.15%). Out of 54 patients who underwent thymectomy, HT group had the highest rate of thymectomy (13/16 patients, 81.25%).
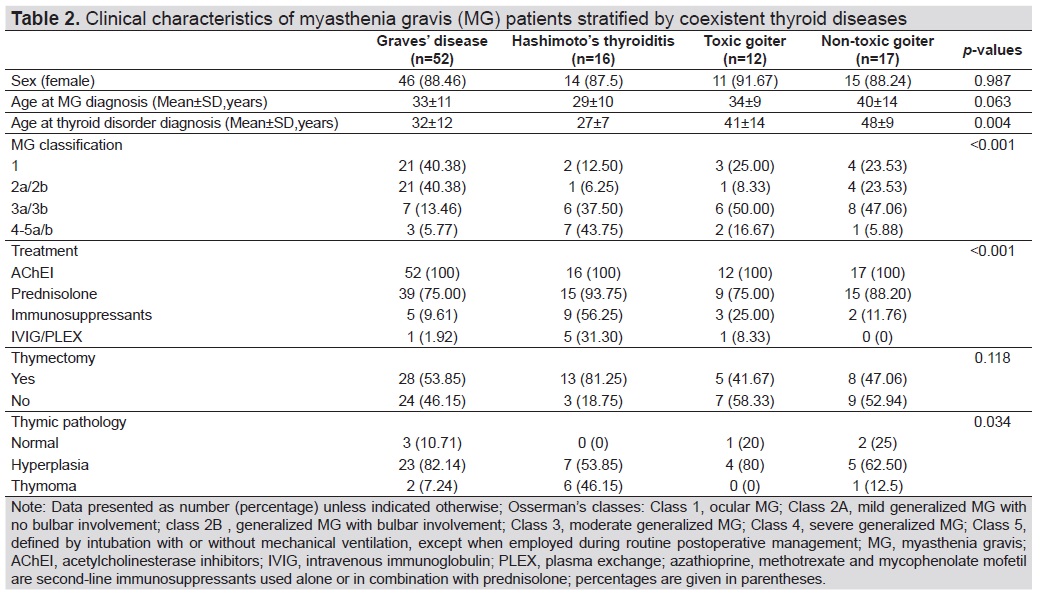
Table 2. Clinical characteristics of myasthenia gravis (MG) patients stratified by coexistent thyroid diseases
MG is an autoimmune neuromuscular disorder due to a defective transmission of the nerve impulse to muscles, causing muscle weakness and abnormal fatigability. The coexistence of other autoimmune diseases in MG is well recognized,[7],[10],[13],[14] including AITD[7],[12],[13],[15],[16],[17],[18],[19],[20] which is an endocrine disease characterized by the development of autoimmunity against thyroid antigens. The two main AITDs are Graves’ disease (GD) and Hashimoto’s thyroiditis (HT) which are the most common diseases coexisting with MG, with a frequency of 7% and 3%.[17] In the present study, the rate of MG patients with thyroid disorders were 0.99/1,000 which was comparable with the general population (0.01%).[4],[21] The rate of MG was higher in those with AITD (1.80 and 9.92/1,000 in GD and HT, respectively). Unlike the previous studies, we found that the rate of MG in HT was higher than GD (Table 1). Not surprising, female sex accounted for almost 90% of patients with MG in all types of thyroid diseases (Table 2).[11],[12] Note that MG coexisting with HT also had younger age at diagnosis of HT than other groups of thyroid disorders.
We also found that MG coexisting with HT was quite severe, required more aggressive treatments, and was more related with thymoma (Table 2) than other types of thyroid disorders including GD. Over 80% of MG patients have thymic abnormalities, including hyperplasia and thymoma.[22],[23] Several previous studies have analyzed the incidence of thymoma in MG with AITDs, but the estimates vary widely across studies because of differences in study populations and diagnostic criteria. A Japanese study demonstrated a greater frequency of thymic hyperplasia in MG patients with AITDs,[24] but not in the Chinese or Italian study.[8],[25] Thymic status was available for about 56% (54/97) of patients in this current study; mostly in GD group. The study showed a greater number with thymic hyperplasia in MG patients with GD and a greater number of thymomas in MG patients with HT. Since thymectomy in MG patients usually tends to be performed in those patients with thymoma or a more severe status, this would result in selection bias. The reason why MG with HT in this study was more severe and more common than the GD group may be due to different circulating thyroid antibodies in HT and GD.[20],[26],[27],[28] The TPOAb and TgAb are primary thyroid antibodies in HT, while the TRAb is primarily seen in GD.[28] The differences in thyroid antibodies may lead to different clinical manifestation of MG. GD with MG had more patients with ocular MG (40.38%) than other thyroid disorders (Table 2).
From the results of this study, there are two clinical implications. First, patients with ocular MG or mild generalized MG have high prevalence of Graves’ diseases (40.38%). For those with class 3-5 MG or moderate to severe MG, the prevalence of HT or toxic goiter was between 37.50%-50% (Table 2). These patients should be evaluated for thyroid function tests, thyroid antibodies or thyroid scan when appropriate. Second, MG patients with HT may need more aggressive treatment such as immunosuppressive treatment (56.25%) or prednisolone (93.75%) and relate to thymoma (46.15%).
The main limitation of this study is that it is retrospective and not a case–control study. The data were acquired from existing medical records such as summary charts of admitted patients and OPD cards of outpatients. Therefore, some specific details of each patient such as disease severity, treatment outcome or patient compliance might not have been available for analysis. Secondly, the small sample size of the cases studied remains an important factor limiting the interpretation of the results, although attempts were made to minimize these limitations by reviewing all cases in the study period. Finally, HT in this study was diagnosed only in hypothyroidism which resulted in markedly low prevalence than the GD group.
MG is most prevalent in patients with HT. Patients with both MG and HT had more severe MG status and had higher rate of thymoma.
ACKNOWLEDGMENTSThe authors express their deep gratitude to Assoc. Prof. Dr. Somsak Tiamkao and Dr.Suranut Charoensri, their research supervisors, for their enthusiastic encouragement, useful and constructive recommendations on this project. Special thanks given to Emeritus Prof. Athasit Vejjajiva for his professional guidance and valuable support. Assistance with the statistics provided by Mr. Suthipol Udompunturak was greatly appreciated. The authors also acknowledge Prof. James Arthur Will for editing the MS via Publication Clinic KKU, Thailand.
Statement of AuthorshipAll authors certified fulfillment of ICMJE authorship criteria.
Author DisclosureThe authors declared no conflict of interest
Funding SourceFinancial support was provided by the Neuroscience Research and Development Group, Khon Kaen University, Thailand.
[1] Patrick J, Lindstrom J. Autoimmune response to acetylcholine receptor. Science. 1973;180(4088):871-2.
[2] Hoch W, McConville J, Helms S, Newsom-Davis J, Melms A, Vincent A. Auto-antibodies to the receptor tyrosine kinase MuSK in patients with myasthenia gravis without acetylcholine receptor antibodies. Nat Med. 2001;7(3):365-8.
[3] Conti-Fine BM, Milani M, Kaminski HJ. Myasthenia gravis: Past, present, and future. J Clin Invest. 2006;116(11):2843-54.
[4] Phillips LH 2nd. The epidemiology of myasthenia gravis. Ann N Y Acad Sci. 2003;998:407-12.
[5] Grob D, Brunner N, Namba T, Pagala M. Lifetime course of myasthenia gravis. Muscle Nerve. 2008;37(2):141-9.
[6] Viegas S, Vincent A. Chapter 54 - Myasthenia Gravis and Related Disorders. In: Rose NR, Mackay IR, editors. The Autoimmune Diseases (Fifth Edition). Boston: Academic Press; 2014.
[7] Rennie GE. Exopthalmic goitre combined with myasthenia gravis. Rev Neurol Psychiatry. 1908;6:229-33.
[8] Marinó M, Ricciardi R, Pinchera A, et al. Mild clinical expression of myasthenia gravis associated with autoimmune thyroid diseases. J Clin Endocrinol Metab. 1997;82(2):438-43.
[9] Mamarabadi M, Razjouyan H, Moghaddasi M. Hypothyroidism, the main thyroid dysfunction in Iranian patients with myasthenia gravis: A case serie. Iran. J. Neurol. 2011;10(1-2):22-5.
[11] Kiessling WR, Finke R, Kotulla P, Schleusener H. Circulating TSH-binding inhibiting immunoglobulins in myasthenia gravis. Acta Endocrinol (Copenh). 1982;101(1):41-6.
[12] Peacey SR, Belchetz PE. Graves' disease: Associated ocular myasthenia gravis and a thymic cyst. J R Soc Med. 1993;86(5):297-8.
[13] Drachman DB. Myasthenia Gravis and the thyroid gland. N Engl J Med. 1962;266(7):330-3.
[14] GGalbraith RF, Summerskill WH, Murray J. Systemic lupus
erythematosus, cirrhosis and ulcerative colitis after thymectomy for
myasthenia gravis. N Engl J Med. 1964;270:229-32.
[15] Garlepp MJ, Dawkins RL, Christiansen FT, et al. Autoimmunity in ocular and generalised myasthenia gravis. J Neuroimmunol. 1981;1(3):325-32.
[16] Tola MR, Caniatti LM, Casetta I, et al. Immunogenetic heterogeneity and associated autoimmune disorders in myasthenia gravis: A population-based survey in the province of Ferrara, northern Italy. Acta Neurol Scand. 1994;90(5):318-23.
[17] Thorlacius S, Aarli JA, Riise T, Matre R, Johnsen HJ. Associated disorders in myasthenia gravis: Autoimmune diseases and their relation to thymectomy. Acta Neurol Scand. 1989;80(4):290-5.
[18] Scherbaum WA, Schumm F, Maisch B, et al. Myasthenia gravis: Overlap with 'polyendocrine' autoimmunity. Klin Wochenschr. 1983;61(10):509-15.
[19] Aarli JA, Gilhus NE, Matre R. Myasthenia gravis with thymoma is not associated with an increased incidence of non-muscle autoimmune disorders. Autoimmunity. 1992;11(3):159-62.
[20] Christensen PB, Jensen TS, Tsiropoulos I, et al. Associated autoimmune diseases in myasthenia gravis. A population-based study. Acta Neurol Scand. 1995;91(3):192-5.
[21] Meriggioli MN, Sanders DB. Autoimmune myasthenia gravis: Emerging clinical and biological heterogeneity. Lancet Neurol. 2009;8(5):475-90. .
[22] Juel VC, Massey JM. Myasthenia gravis. Orphanet J Rare Dis. 2007;2:44.
[23] Cavalcante P, Bernasconi P, Mantegazza R. Autoimmune mechanisms in myasthenia gravis. Curr Opin Neurol. 2012;25(5):621-9.
[24] Kanazawa M, Shimohata T, Tanaka K, Nishizawa M. Clinical features of patients with myasthenia gravis associated with autoimmune diseases. Eur J Neurol. 2007;14(12):1403-4.
[25] Chen YP, Wei DN, Chen B. [The clinical features of myasthenia gravis associated with thyroid abnormalities]. Zhonghua Nei Ke Za Zhi. 2010;49(7):602-5.
[26] Mao ZF, Yang LX, Mo XA, et al. Frequency of autoimmune diseases in myasthenia gravis: a systematic review. Int J Neurosci. 2011;121(3):121-9.
[27] Klein R, Marx A, Ströbel P, Schalke B, Nix W, Willcox N. Autoimmune associations and autoantibody screening show focused recognition in patient subgroups with generalized myasthenia gravis. Hum Immunol. 2013;74(9):1184-93.
[28] Lopomo A, Berrih-Aknin S. Autoimmune thyroiditis and myasthenia gravis. Front Endocrinol (Lausanne). 2017;8:169.
PubMed
PubMed Central
CrossRef
Authors are required to accomplish, sign and submit scanned copies of the JAFES Author Form consisting of: (1) Authorship Certification, that all the requirements for authorship have been met by each author, and that the final version of the manuscript has been read and approved by all authors; (2) the Author Declaration, that the article represents original material that is not being considered for publication or has not been published or accepted for publication elsewhere; (3) the Statement of Copyright Transfer [accepted manuscripts become the permanent property of the JAFES and are licensed with an Attribution-Share Alike-Non-Commercial Creative Commons License. Articles may be shared and adapted for non-commercial purposes as long as they are properly cited]; and the ICMJE form for Disclosure of Potential Conflicts of Interest. For original articles, authors are required to submit a scanned copy of the Ethics Review Approval of their research as well as registration in trial registries as appropriate. For manuscripts reporting data from studies involving animals, authors are required to submit a scanned copy of the Institutional Animal Care and Use Committee approval. For Case Reports or Series, and Images in Endocrinology, consent forms, are required for the publication of information about patients; otherwise, appropriate ethical clearance has been obtained from the institutional review board. Articles and any other material published in the JAFES represent the work of the author(s) and should not be construed to reflect the opinions of the Editors or the Publisher.