Lyza Camille P. Gadong, MD, FPCP
Section of Endocrinology, Diabetes and Metabolism
Department of Internal Medicine, Makati Medical Center
2 Amorsolo Street, Legaspi Village, Makati City, Philippines 1229
Tel. No.: +632 888-8999
E-mail: mmc@makatimedi.net.ph
ORCiD: https://orcid.org/0000-0001-5517-9990
e-ISSN 2308-118x
Printed in the Philippines
Copyright © 2019 by the JAFES
Received April 18, 2019. Accepted June 16, 2019.
Published Online First: November 9, 2019.
The cell origin, histopathologic features, and prognosis of medullary and papillary thyroid carcinoma are different and to have them occur simultaneously in a single patient is a rare occurrence.
This is a case of a 38-year-old female who presented with an enlarging anterior neck mass whose fine needle aspiration biopsy could not rule out a papillary lesion. Thus, she was advised to undergo total thyroidectomy, and her final histopath showed a simultaneous medullary and papillary thyroid carcinoma. Her initial serum calcitonin was elevated at 252 pg/ ml, and it remained persistently elevated over the course of 7 months. A repeat ultrasound revealed solid nodules with coarse calcifications and enlarged lymph nodes at both submandibular regions. This warranted a repeat surgery with neck dissection with the finding of eight lymph nodes positive for metastatic carcinoma. On follow up after her second surgery, the calcitonin decreased to 42.70 pg/ml.
Knowledge of this simultaneous occurrence of medullary thyroid carcinoma and papillary cancer is important for its prognostic implications and therapeutic plan.
Keywords: thyroid cancer, papillary thyroid cancer, medullary thyroid cancerThe cell origin, histopathologic features, and prognosis of medullary thyroid carcinoma and papillary thyroid carcinoma are different and to have them occur simultaneously in a single patient is a rare event. Data about the simultaneous occurrence of these tumors are based mainly on case reports and series.
This is a case of a 38-year-old female who has simultaneous medullary thyroid cancer in the left thyroid lobe and papillary thyroid cancer in the right thyroid lobe. She was diagnosed to have thyrotoxicosis at 18 years old presenting with palpitations and an anterior neck mass. She was maintained on an unrecalled dose of carbimazole and propanolol for 1 year which was eventually discontinued since her thyroid function test normalized, with noted decrease in size of her anterior neck mass.
Five years prior, she began noticing an enlarging anterior neck mass. There was no associated hoarseness of voice, dysphagia, weight loss, palpitations or difficulty of breathing. She consulted an endocrinologist and work up was done. Thyroid function test showed an elevated TSH at 11.84 uIU/ml and a normal FT4 at 15 uIU/ml. Her ultrasound showed an enlarged left thyroid gland with a 2.38 x 1.4 x 1.69 solid nodule with calcifications and minimal hypervascularity. Few coarse parenchymal calcifications are noted at the mid portion of the right lobe. She underwent fine needle aspiration biopsy (FNAB) of her left thyroid lobe with a result of an adenomatous goiter with concomitant lymphocytic thyroiditis. She was given Levothyroxine 50 mcg daily with serial monitoring of her thyroid function test and ultrasound.
Repeat ultrasound two years later showed interval increase in the size of the previously noted solid nodule with calcifications in the left lobe, with new nodules noted on the right lobe. This warranted a repeat biopsy. Repeat ultrasound guided fine needle aspiration biopsy of the nodule on the right thyroid gland showed nodular hyperplasia but cannot rule out papillary lesion. While the FNAB of the nodule on the left showed nodular hyperplasia of left thyroid lobe. Thus, she underwent total thyroidectomy.
Microscopic examination of the isthmus and left lobe were consistent with medullary carcinoma, with the tumor size 6 cm in its greatest dimension. The right lobe showed papillary thyroid carcinoma, with the tumor size in greatest dimension 1.1 cm, with no lymphovascular or perineural invasion (Figure 1). Immunohistochemistry report for the isthmus and left lobe were chromogranin positive, calcitonin positive supporting the diagnosis of medullary thyroid carcinoma.
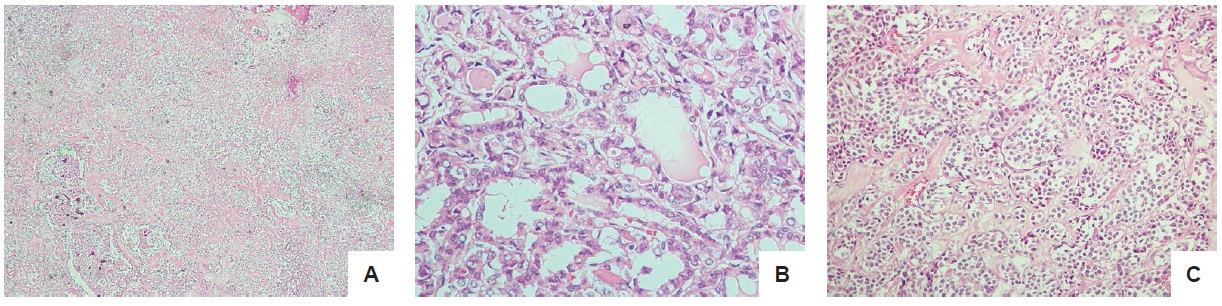
Figure 1. Histopath. (A) Areas of normal parenchyma, thyroiditis, and papillary carcinoma (H&E, 40x). (B) Papillary Thyroid carcinoma, right lobe. Overlapping clear ground glass nuclei with eosinophilic inclusions (H&E, 400x). (C) Medullary carcinoma. Cells with eccentrically located round nuclei with salt and pepper nuclear chromatin pattern (H&E, 100x).
After the surgery, serum thyroglobulin was 0.307 ng/ml with normal antithyroglobulin 90.859 IU/ml, while serum calcitonin was elevated at 252 pg/ ml. The elevated serum calcitonin warranted a work up for possible metastasis. Thus, a neck ultrasound was requested which showed post thyroidectomy. Solid nodules with coarse calcifications, left parajugular region. Enlarged lymph nodes, both submandibular regions (Figure 2).
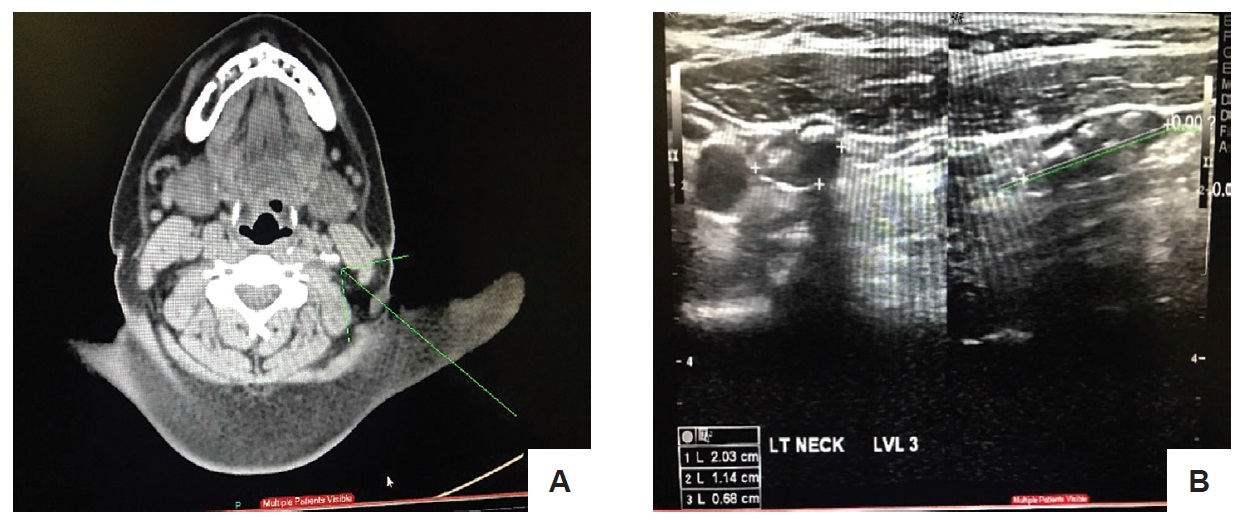
Figure 2. (A) CT scan of the neck with contrast. Calcification in the left infrahyoid carotid space possibly calcified lymph nodes. (B) Neck ultrasound. Nodes with calcifications in the left parajugular region (level 3).
Findings on CT scan of the neck, chest and whole abdomen were nonspecific. CT scan of the neck revealed no evidence of cervical lymphadenopathy (Figure 2).
Chest CT scan showed nonspecific nodules along the left lung fissure and along the posterior hemithoraces. Whole abdomen CT scan showed mild hepatosplenomegaly, uterine myoma and Nabothian cyst. For further work- up, a whole-body fluorodeoxyglucose PET scan was requested. Results showed no hypermetabolic malignant looking disease in the neck and elsewhere. The mildly hypermetabolic calcified left submandibular and left supraclavicular lymph nodes look inflammatory/reactive.
However, serum calcitonin on serial monitoring was persistently elevated (362, 197 pg/ml) over the course of 7 months (Table 1). A repeat thyroid ultrasound showed solid nodules (4) with coarse calcifications at the left parajugular area (level 3).
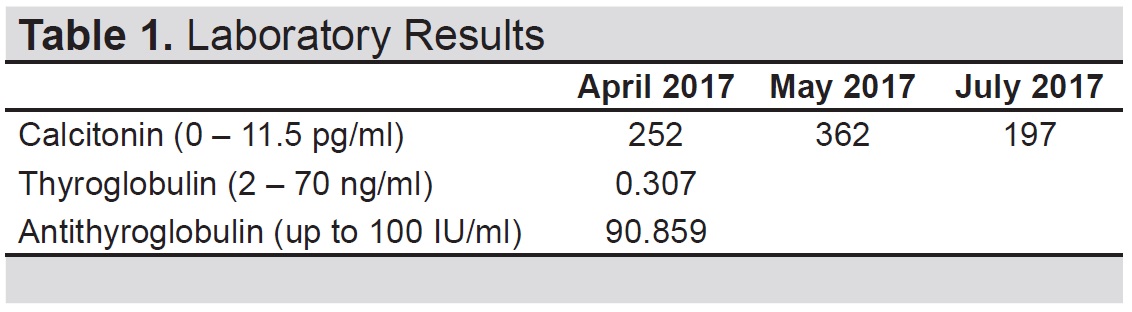
Table 1. (A) Laboratory results
Thus, she underwent neck exploratory dissection, with the finding of eight of twenty-five lymph nodes with metastatic carcinoma. On follow up three months after the operation, the calcitonin decreased to 42.70 pg/ ml. Nature and prognosis of papillary and medullary thyroid carcinoma were explained to the patient, with plans of monitoring for recurrence. Medullary thyroid carcinoma can occur sporadically or it may be associated with hereditary syndromes such as Multiple Endocrine Neoplasia Type 2. Thus, molecular studies investigating RET germline mutations in exons 11, 15 and 16 were performed, and patient was found to be positive for a mutation in exon 15. She is now recommended for work up for pheochromocytoma and hyperparathyroidism to screen for multiple endocrine neoplasia 2 syndromes.
Papillary thyroid carcinoma (PTC) is the most common type of thyroid carcinoma (90%), and it originates from the follicular cells of the endoderm. Medullary thyroid carcinoma, on the other hand, arises from the parafollicular C cells of the ultimobranchial body of the neural crest, and it represents only 5% of all thyroid cancers.[1]
The simultaneous occurrence of an independent medullary thyroid carcinoma, a tumor showing morphological features of medullary thyroid carcinoma (MTC) with positive immunoreactive calcitonin cells, along with papillary carcinomas in the same thyroid gland is uncommon, but it has been reported.
Although the simultaneous occurrence of papillary and medullary thyroid carcinoma in one patient may be entirely coincidental, several authors have tried to explain as to why it happens. According to Erhamamci (2014), the simultaneous occurrence of MTC and DTC in the same thyroid gland can occur in two ways: as a mixed tumor showing dual differentiation or as a collision tumor showing two separate different carcinomas.[2] They concluded that out of 1420 patients, 0.28% of patients with DTC displayed simultaneous MTC.[2] Machens (2011) followed 1019 patients diagnosed with papillary thyroid carcinoma, and found 26 patients had simultaneous MTC with a prevalence rate of 2.6.[3]
Previous studies have also mentioned several other theories about this concurrence. The first is the ‘stem cell theory’ which describes a common stem cell that transform into both follicular and C-cell lineages.[4] Another is the ‘field effect theory’ which proposes that simultaneous transformation of both follicular and C cells is a result of common neoplastic stimuli. On the other hand, the ‘collision theory’ suggests that two independent tumours are located in the same thyroid by simple coincidence.[4]
Papillary carcinoma generally has a good prognosis since most patients are diagnosed during the early stage of the disease (stage I or II), with 5% having a lethal outcome.[5] Medullary thyroid carcinoma, on the other hand, may have an indolent clinical course or an aggressive variant with a high mortality rate.[5] Distant metastases are present in 13% of patients at initial diagnosis and portend a poor prognosis, with a 10-year survival rate of only 40%.[5] MTC is difficult to cure with a high recurrence rate of 50%.[5] According to WHO, the prognosis of mixed medullary and follicular thyroid carcinoma depends upon the medullary component. Thus, the presence of the medullary component makes the prognosis worse as compared to a pure papillary carcinoma. Patients who have clinically evident disease are best treated with a minimum of a total thyroidectomy and bilateral central neck dissection.[5] The use of adjuvant radioiodine therapy for the papillary carcinoma component of this disease has been mentioned in several case reports with good results. In a study by Diogini et al. (2007), they presented a 65-year-old man with multicentric PTC and MTC associated with diffuse lymphocytic-type thyroiditis who underwent total thyroidectomy with neck dissection and subsequent radioiodine treatment.5 This patient had normal thyroglobulin and calcitonin levels on follow up.[5]
Knowledge of this simultaneous occurrence of medullary thyroid carcinoma and papillary cancer is important because of the poor prognosis associated with medullary thyroid carcinoma. This report also highlights the importance of immunohistochemical markers to make a correct diagnosis.
Ethical ConsiderationPatient consent was obtained before submission of the manuscript.
Statement of AuthorshipAll authors certified fulfillment of ICMJE authorship criteria.
Author DisclosureThe authors declared no conflict of interest
Funding SourceNone.
[1] Katoh H, Yamashita K, Enomoto T, Watanabe M. Classification and general considerations of thyroid cancer. Ann Clin Pathol. 2015;3:1045.
[2] Erhamamci S, Reyhan M, Koçer NE, Nursal GN, Torun N, Yapar AF. Simultaneous occurrence of medullary and differentiated thyroid carcinomas. Report of 4 cases and brief review of the literature. Hell J Nucl Med. 2014;17(2):148-52.
[3] Machens A, Dralle H. Simultaneous medullary and papillary thyroid cancer: a novel entity? Ann Surg Oncol. 2012;19(1):37-44.
[4] Adnan Z, Arad E, Dana J, Shendler Y, Baron E. Simultaneous occurrence of medullary and papillary thyroid microcarcinomas: A case series and review of the literature. J Med Case Rep. 2013;7:26.
[5] Dionigi G, Castano P, Bertolini V, et al. Simultaneous medullary and papillary thyroid cancer: two case reports. J Med Case Rep. 2007;1:133.
[6] Al Awan I, M K, Amir 1st, et al. Turner syndrome genotype and
phenotype and their effect on presenting features and timing of
diagnosis. Int J Health Sci (Qassim). 2014;8(2):195-202.
[7] Akbaş E, Yazıcı FG, Durukan H, Topal H, Erdoğan NE. Cytogenetic
and clinical evaluation of two cases that have 45,X/46,X,i(Xq) and
46,X,i(Xq) karyotype 45,X/46,X,i(Xq) ve 46,X,i(Xq). J Clin Exp Invest.
2014; 5(3):444-8.
[8] Muntaj S, Ganale FA, Purva SV, Radhika S, Tilak P. Karyotypic
variables in Turner syndrome: A case series. Int J Sci Study.
2015;3(4):171-5.
[9] Gravholt CH. Epidemiological, endocrine and metabolic features
in Turner syndrome. Eur J Endocrinol. 2004:151(6):657–87.
[10] Haber HP, Ranke MB. Pelvic ultrasonography in Turner syndrome:
Standards for uterine and ovarian volume. J Ultrasound Med.
1999;18(4):271-6.
[11] Tebbe B, Golinick H, Müller R, Reupke HJ, Orfanos CE. Alopecia
areata and diffuse hypotrichosis associated with Ullrich-Turner
syndrome. Presentation of 4 patients. Hautarzt. 1993;44(10):647-52.
[12] Naeraa RW, Gravholt CH, Hansen J, Nielsen J, Juul S. Mortality in
Turner syndrome. In: Albertsson-Wikland K, Ranke MB, eds. Turner
syndrome in a lifespan perspective: Research and clinical aspects.
Amsterdam: Elsevier, 1995.
[13] Miller MJ, Geffner ME, Lippe BM, et al. Echocardiography reveals
a high incidence of bicuspid aortic valve in Turner syndrome. J
Pediatr.1983;102(1):47–50.
[14] Gøtzsche CO, Krag-Olsen B, Nielsen J, Sørensen KE, Kristensen BO.
Prevalence of cardiovascular malformations and association with
karyotypes in Turner’s syndrome. Arch Dis Child. 1994;71(5):433-6.
[15] Ross JL, Feuillan P, Long LM, Kowal K, Kushner H, Cutler GB Jr.
Lipid abnormalities in Turner syndrome. J Pediatr. 1995;126(2):242–5.
[16] Sylvén L, Hagenfeldt K, Bröndum-Nielsen K, von-Schoultz B. Middleaged
women with Turner’s syndrome. Medical status, hormonal
treatment, and social life. Acta Endocrinol (Copenh). 1991;125(4):359–
65.
[17] Radetti G, Mazzanti L, Paganini C, et al. Frequency, clinical
and laboratory features of thyroiditis in girls with Turner’s
syndrome. The Italian Study Group for Turner’s Syndrome.
Acta Paediatr. 1995;84(8):909–12.
[18] Elsheikh M, Wass JA, Conway GS. Autoimmune thyroid syndrome
in women with Turner’s syndrome—the association with karyotype.
Clin Endocrinol (Oxf). 2001;55(2):223–6.
[19] Bilge I, Kayserili H, Emre S, et al. Frequency of renal malformations
in Turner syndrome: Analysis of 82 Turkish children. Pediatr
Nephrol. 2000;14(12):1111-4.
[20] RSculerati N, Oddoux C, Clayton CM, Lim JW, Oster H. Hearing loss
in Turner syndrome. Laryngoscope. 1996;106(8):992–7.
[21] Bondy CA. New issues in the diagnosis and management of Turner
syndrome. Rev Endocr Metab Disord. 2005;6(4):269-80.
[22] Turner Syndrome. In Gardner D, Shoback D, eds. Greenspan’s
basic and clinical endocrinology (Lange Medical Books),8th ed.
Philadelphia: McGraw-Hill Medical, 2007.
[23] Gomez-Lobo, V, Amies Oelschlager AM; North American Society
for Pediatric and Adolescent Gynecology. Disorders of sexual
development in adult women. Obstet Gynecol. 2016;128(5):1162-
73.
PubMed PubMed Central CrossRef
Authors are required to accomplish, sign and submit scanned copies of the JAFES Author Form consisting of: (1) Authorship Certification, that all the requirements for authorship have been met by each author, and that the final version of the manuscript has been read and approved by all authors; (2) the Author Declaration, that the article represents original material that is not being considered for publication or has not been published or accepted for publication elsewhere; (3) the Statement of Copyright Transfer [accepted manuscripts become the permanent property of the JAFES and are licensed with an Attribution-Share Alike-Non-Commercial Creative Commons License. Articles may be shared and adapted for non-commercial purposes as long as they are properly cited]; and the ICMJE form for Disclosure of Potential Conflicts of Interest. For original articles, authors are required to submit a scanned copy of the Ethics Review Approval of their research as well as registration in trial registries as appropriate. For manuscripts reporting data from studies involving animals, authors are required to submit a scanned copy of the Institutional Animal Care and Use Committee approval. For Case Reports or Series, and Images in Endocrinology, consent forms, are required for the publication of information about patients; otherwise, appropriate ethical clearance has been obtained from the institutional review board. Articles and any other material published in the JAFES represent the work of the author(s) and should not be construed to reflect the opinions of the Editors or the Publisher.