The Effect of DPP4 Inhibitor on Glycemic Variability in Patients with Type 2 Diabetes treated with twice-daily Premixed Human Insulin
DOI:
https://doi.org/10.15605/jafes.036.02.11Keywords:
glycemic variability, dipeptidyl peptidase 4 inhibitors, premixed human insulin, continuous glucose monitoring, type 2 diabetes mellitusAbstract
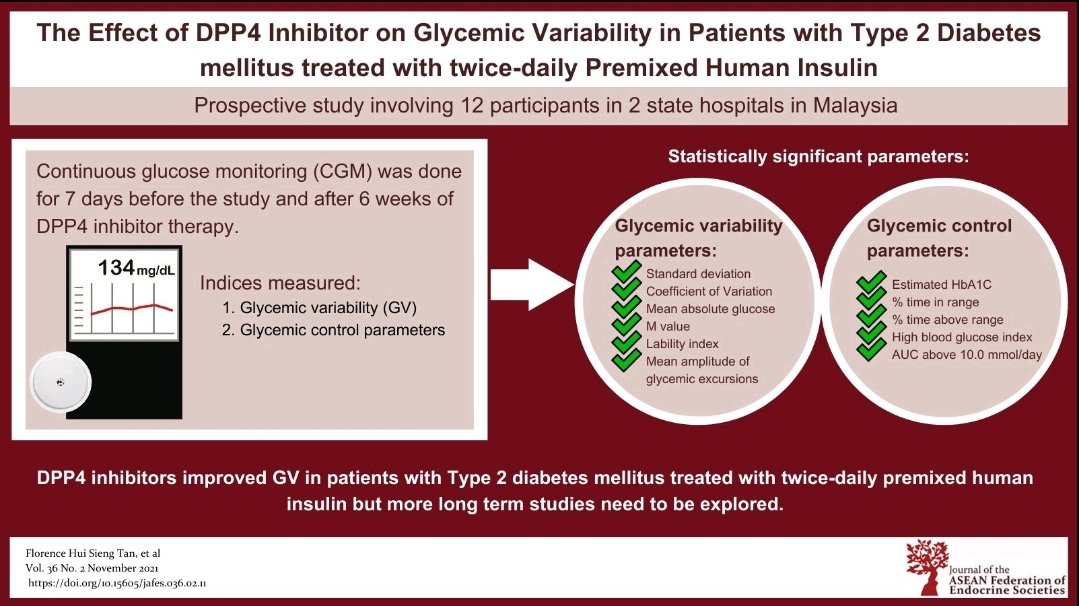
Objective. To evaluate the effect of adding DPP4 inhibitor (DPP4-I) on glycemic variability (GV) in patients with type 2 diabetes mellitus (T2DM) treated with premixed human insulin (MHI).
Methodology. We conducted a prospective study in patients with T2DM on twice-daily MHI with or without metformin therapy. Blinded continuous glucose monitoring was performed at baseline and following 6 weeks of Vildagliptin therapy.
Results: Twelve patients with mean (SD) age of 55.8 (13.1) years and duration of disease of 14.0 (6.6) years were recruited. The addition of Vildagliptin significantly reduced GV indices (mmol/L): SD from 2.73 (IQR 2.12-3.66) to 2.11 (1.76-2.55), p=0.015; mean amplitude of glycemic excursions (MAGE) 6.94(2.61) to 5.72 (1.87), p=0.018 and CV 34.05 (8.76) to 28.19 (5.36), p=0.010. In addition, % time in range (3.9-10 mmol/l) improved from 61.17 (20.50) to 79.67 (15.33)%, p=0.001; % time above range reduced from 32.92 (23.99) to 18.50 (15.62)%, p=0.016; with reduction in AUC for hyperglycemia from 1.24 (1.31) to 0.47 (0.71) mmol/day, p=0.015. Hypoglycemic events were infrequent and the reduction in time below range and AUC for hypoglycemia did not reach statistical significance.
Conclusion. The addition of DPP4-I to commonly prescribed twice-daily MHI in patients with T2DM improves GV and warrants further exploration.
Downloads
References
Frontoni S, Di Bartolo P, Avogaro A, Bosi E, Paolisso G, Ceriello A. Glucose variability: An emerging target for the treatment of diabetes mellitus. Diabetes Res Clin Pract. 2013;102(2):86-95. https://pubmed.ncbi.nlm.nih.gov/24128999. https://doi.org/10.1016/j.diabres.2013.09.007.
Rayman G. Glycaemic control, glucose variability and the triangle of diabetes care. Br J Diabetes. 2016(Suppl 1);16:S3-6. https://doi.org/10.15277/bjd.2016.070.
Kovatchev BP. Metrics for glycaemic control - from HbA1c to continuous glucose monitoring. Nat Rev Endocrinol. 2017;13(7):425-36. https://pubmed.ncbi.nlm.nih.gov/28304392. https://doi.org/10.1038/nrendo.2017.3.
Rama Chandran S, Tay WL, et al. Beyond HbA1c: Comparing glycemic variability and glycemic indices in predicting hypoglycemia in type 1 and type 2 diabetes. Diabetes Technol Ther. 2018;20(5):353-62. https://pubmed.ncbi.nlm.nih.gov/29688755. https://doi.org/10.1089/dia.2017.0388.
Zinman B, Marso SP, Poulter NR, et al. Day-to-day fasting glycaemic
variability in DEVOTE: Associations with severe hypoglycaemia and
cardiovascular outcomes (DEVOTE 2). Diabetologia. 2018;61(1):48–57. https://pubmed.ncbi.nlm.nih.gov/28913575. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6002963. https://doi.org/10.1007/s00125-017-4423-z.
Ceriello A, Monnier L, Owens D. Glycaemic variability in diabetes: Clinical and therapeutic implications. Lancet Diabetes Endocrinol. 2019;7(3):221-30. https://pubmed.ncbi.nlm.nih.gov/30115599. https://doi.org/10.1016/S2213-8587(18)30136-0.
Gorst C, Kwok CS, Aslam S, et al. Long-term glycemic variability and risk of adverse outcomes: A systematic review and meta-analysis. Diabetes Care. 2015;38(12):2354–69. https://pubmed.ncbi.nlm.nih.gov/26604281. https://doi.org/10.2337/dc15-1188.
Jung HS. Clinical implications of glucose variability: Chronic complications of diabetes. Endocrinol Metab (Seoul). 2015;30(2):167-74. https://pubmed.ncbi.nlm.nih.gov/26194076. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4508260. https://doi.org/10.3803/EnM.2015.30.2.167.
Ceriello A, Kilpatrick ES. Glycemic variability: Both sides of the story. Diabetes Care. 2013; 36(Suppl 2): S272–5. https://pubmed.ncbi.nlm.nih.gov/23882058. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3920802. https://doi.org/10.2337/dcS13-2030.
Kalra S, Balhara YP, Sahay BK, et al. Why is premixed insulin the preferred insulin? Novel answers to a decade-old question. J Assoc Physicians India. 2013;61(Suppl 1):9-11. https://pubmed.ncbi.nlm.nih.gov/24482980.
Kong APS, Lew T, Lau ESH, et al. Real-world data reveal unmet clinical needs in insulin treatment in Asian people with type 2 diabetes: The Joint Asia Diabetes Evaluation (JADE) Register. Diabetes Obes Metab. 2020;22(4):669‐79. https://pubmed.ncbi.nlm.nih.gov/31903728. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7540442. https://doi.org/10.1111/dom.13950.
Rizvi AA. Treatment of type 2 diabetes with biphasic insulin analogues. Eur Med J Diabetes. 2016;4(1):74-83. https://pubmed.ncbi.nlm.nih.gov/27918600. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5134918.
Boehm BO, Home PD, Behrend C, Kamp NM, Lindholm A. Premixed insulin aspart 30 vs. premixed human insulin 30/70 twice daily: A randomized trial in Type 1 and Type 2 diabetic patients. Diabet Med. 2002;19(5):393-9.https://pubmed.ncbi.nlm.nih.gov/12027927. https://doi.org/10.1046/j.1464-5491.2002.00733.x.
Guerci B, Monnier L, Serusclat P, et al. Continuous glucose profiles with vildagliptin versus sitagliptin in add-on to metformin: Results from the randomized Optima study. Diabetes Metab. 2012;38(4):359–66. https://pubmed.ncbi.nlm.nih.gov/22809630. https://doi.org.10.1016/j.diabet.2012.06.001.
Rizzo MR, Barbieri M, Marfella R, Paolisso G. Reduction of oxidative stress and inflammation by blunting daily acute glucose fluctuations in patients with type 2 diabetes: Role of dipeptidyl peptidase-IV inhibition. Diabetes Care. 2012;35(10):2076-82. https://pubmed.ncbi.nlm.nih.gov/22688551. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3447848. https://doi.org/10.2337/dc12-0199.
Kim NH, Kim DL, Kim KJ, et al. Effects of vildagliptin or pioglitazone on glycemic variability and oxidative stress in patients with type 2 diabetes inadequately controlled with metformin monotherapy: A 16-week, randomised, open label, pilot study. Endocrinol Metab (Seoul). 2017;32(2):241–7. https://pubmed.ncbi.nlm.nih.gov/28685513. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5503869. https://doi.org/10.3803/EnM.2017.32.2.241.
Kim HS, Shin JA, Lee SH, et al. A comparative study of the effects of a dipeptidyl peptidase-IV inhibitor and sulfonylurea on glucose variability in patients with type 2 diabetes with inadequate glycemic control on metformin. Diabetes Technol Ther. 2013;15:810-6. https://pubmed.ncbi.nlm.nih.gov/24050737. https://doi.org/10.1089/dia.2013.0038.
Li FF, Shen Y, Sun R, et al. Effects of vildagliptin add-on insulin therapy on nocturnal glycemic variations in uncontrolled type 2 diabetes. Diabetes Ther 2017;8(5):1111–22. https://pubmed.ncbi.nlm.nih.gov/28921310. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5630558. https://doi.org/10.1007/s13300-017-0303-2.
Dupont WD, Plummer WD. Power and sample size calculations: A review and computer program. Control Clin Trials. 1990;11(2):116-28. https://pubmed.ncbi.nlm.nih.gov/2161310. https://doi.org/10.1016/0197-2456(90)90005-m.
American Diabetes Association. Pharmacologic approaches to glycemic
treatment: Standards of medical care in diabetes - 2020. Diabetes Care. 2020;43(Suppl 1):S98–110. https://pubmed.ncbi.nlm.nih.gov/31862752. https://doi.org/10.2337/dc20-S009.
Mosenzon O, Raz I. Intensification of insulin therapy for type 2 diabetic patients in primary care: Basal-bolus regimen versus premix insulin analogs: when and for whom? Diabetes Care. 2013;36(Suppl 2):S212-8. https://pubmed.ncbi.nlm.nih.gov/23882048. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3920792. https://doi.org/10.2337/dcS13-2007.
Davidson JA, Liebl A, Christiansen JS, et al. Risk for nocturnal hypoglycemia with biphasic insulin aspart 30 compared with biphasic human insulin 30 in adults with type 2 diabetes mellitus: A meta-analysis. Clin Ther. 2009;31(8):1641-51. https://pubmed.ncbi.nlm.nih.gov/19808125. https://doi.org/10.1016/j.clinthera.2009.08.011.
Zenari L, Marangoni A. What are the preferred strategies for control of glycaemic variability in patients with type 2 diabetes mellitus? Diab Obes Metabol. 2013;15(Suppl. 2):17–25. https://pubmed.ncbi.nlm.nih.gov/24034516. https://doi.org/10.1111/dom.12143.
Miyoshi H, Nomoto H. The difference between SGLT2 and DPP-4 inhibitors on glucose fluctuation in patients with type 2 diabetes. Br J Res. 2017;4(3):21. https://doi.org/ 10.21767/2394-3718.100021.
Wang N, Yang T, Li J, Zhang X. Dipeptidyl peptidase-4 inhibitors as add-on therapy to insulin in patients with type 2 diabetes mellitus: A meta-analysis of randomized controlled trials. Diabetes Metab Syndr Obes. 2019;12:1513–26. https://pubmed.ncbi.nlm.nih.gov/31692532. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6710543. https://doi.org/10.2147/DMSO.S202024.
Lee S, Lee H, Kim Y, Kim E. Effect of DPP-IV inhibitors on glycemic variability in patients with T2DM: A systematic review and meta-analysis. Sci Rep. 2019;9(1):13296. https://pubmed.ncbi.nlm.nih.gov/31527625. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6746852. https://doi.org/10.1038/s41598-019-49803-9.
Nomoto H, Miyoshi H, Sugawara H, et al. A randomized controlled trial comparing the effects of dapagliflozin and DPP-4 inhibitors on glucose variability and metabolic parameters in patients with type 2 diabetes mellitus on insulin. Diabetol Metab Syndr. 2017;9:54. https://pubmed.ncbi.nlm.nih.gov/28725273. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5514514. https://doi.org/10.1186/s13098-017-0255-8.
He YL, Kulmatycki K, Zhang Y, et al. Pharmacokinetics of vildagliptin in patients with varying degrees of renal impairment. Int J Clin Pharmacol Ther. 2013;51(9):693-703. https://pubmed.ncbi.nlm.nih.gov/23782585. https://doi.org/10.5414/CP201885.
Published
How to Cite
Issue
Section
License
Copyright (c) 2021 Florence Hui Sieng Tan, Chin Voon Tong, Xun Ting Tiong, Bik Kui Lau, Yueh Chien Kuan , Huai Heng Loh, Saravanan Vengadesa Pillai

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
Journal of the ASEAN Federation of Endocrine Societies is licensed under a Creative Commons Attribution-NonCommercial 4.0 International. (full license at this link: http://creativecommons.org/licenses/by-nc/3.0/legalcode).
To obtain permission to translate/reproduce or download articles or use images FOR COMMERCIAL REUSE/BUSINESS PURPOSES from the Journal of the ASEAN Federation of Endocrine Societies, kindly fill in the Permission Request for Use of Copyrighted Material and return as PDF file to jafes@asia.com or jafes.editor@gmail.com.
A written agreement shall be emailed to the requester should permission be granted.









