Lower Plasma Selenoprotein P Levels in Regularly Exercising Young Adults
DOI:
https://doi.org/10.15605/jafes.037.S4Keywords:
mitochondria, physical exercise, reactive oxygen species, selenoprotein PAbstract
Objective. Physical exercise can provide many health benefits in humans. Exercise-induced reactive oxygen species (ROS) formation and its downstream signaling cascades are reported to induce mitochondrial biogenesis in exercising tissues. Selenoprotein P (SELENOP) is the antioxidant hepatokine whose hypersecretion is associated with various metabolic diseases. It was reported to impair exercise-induced reactive oxygen species signaling and inhibit subsequent mitochondrial biogenesis in mice. However, the relationship between selenoprotein P and mitochondrial dynamics in humans has not yet been reported. While reduction of plasma selenoprotein P becomes an attractive therapeutic target for metabolic diseases, the role of regular exercise in this regard is still unknown. This study aimed to analyze the influence of regular habitual exercise on plasma selenoprotein P levels and its association with leucocyte mitochondrial DNA copy number in healthy young adults.
Methodology. Plasma selenoprotein P levels and leucocyte mitochondrial DNA copy numbers were compared in 44 regularly exercising subjects and 44 non-exercising controls, and the correlation between the two parameters was
analyzed. Plasma selenoprotein P levels were measured by Enzyme-linked Immunosorbent Assay, and leucocyte
mitochondrial DNA copy numbers were measured using the qPCR method.
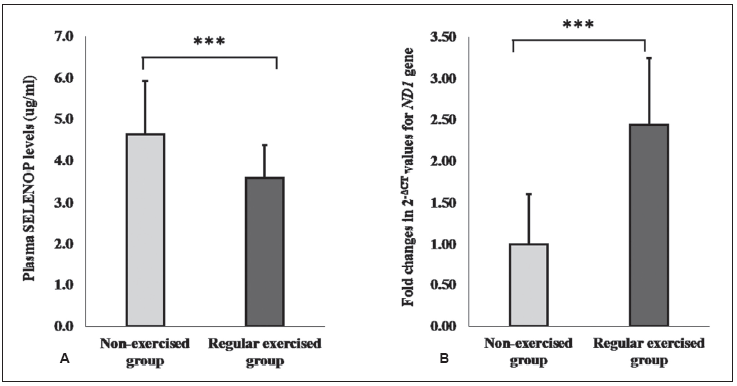
Results. The regular-exercise group had lower plasma selenoprotein P levels with higher leucocyte mitochondrial DNA copy numbers than the non-exercise group. There was a tendency of negative correlation between the two variables in our studied population.
Conclusion. Regular habitual exercise has a beneficial effect on reducing plasma selenoprotein P levels while raising mitochondrial DNA copy numbers.
Downloads
References
Duncan GE, Perri MG, Theriaque DW, Hutson AD, Eckel RH, Stacpoole PW. Exercise training, without weight loss, increases insulin sensitivity and postheparin plasma lipase activity in previously sedentary adults. Diabetes Care. 2003;26(3):557-62. https://pubmed.ncbi.nlm.nih.gov/12610001. https://doi.org/10.2337/diacare.26.3.557.
Braun B, Zimmermann MB, Kretchmer N. Effects of exercise intensity on insulin sensitivity in women with non-insulin-dependent diabetes mellitus. J Appl Physiol. 1995;78(1):300-6. https://pubmed.ncbi.nlm.nih.gov/7713829. https://doi.org/10.1151/jappl.1995.78.1.300.
Booth FW, Tseng BS, Flück M, Carson JA. Molecular and cellular adaptation of muscle in response to physical training. Acta Physiol Scand. 1998;162(3):343-50. https://pubmed.ncbi.nlm.nih.gov/9578380. https://doi.org/10.1046/j.1465-201X.1998.0326e.x.
Powers SK, Wade M, Criswell D, et al. Role of beta-adrenergic mechanisms in exercise training-induced metabolic changes in respiratory and locomotor muscle. Int J Sports Med. 1995;16(1):13-8. https://pubmed.ncbi.nlm.nih.gov/7713624. https://doi.org/10.1055/s-2007-972956.
Philp A, Hargreaves M, Baar K. More than a store: Regulatory roles for glycogen in skeletal muscle adaptation to exercise. Am J Physiol Endocrinol Metab. 2012;302(11):E1343-51. https://pubmed.ncbi.nlm.nih.gov/22395109. https://doi.org/10.1152/ajpendo.00004.2012.
Shi M, Wang X, Yamanaka T, Ogita F, Nakatani K, Takeuchi T. Effects of anaerobic exercise and aerobic exercise on biomarkers of oxidative stress. Environ Health Prev Med. 2007;12(5):202-8. https://pubmed.ncbi.nlm.nih.gov/21432082. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2723376. https://doi.org/10.1265/ehpm.12.202.
He F, Li J, Liu Z, Chuang C, Yang W, Zuo L. Redox mechanism of reactive oxygen species in exercise. Front Physiol. 2016;7:486. https://pubmed.ncbi.nlm.nih.gov/27872595. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5097959. https://doi.org/10.3389/fphys.2016.00486.
Niess AM, Simon P. Response and adaptation of skeletal muscle to exercise--the role of reactive oxygen species. Front Biosci. 2007;12:4826-38. https://pubmed.ncbi.nlm.nih.gov/17569613. https://doi.org/10.2741/2431.
Kang C, O'Moore KM, Dickman JR, Ji LL. Exercise activation of muscle peroxisome proliferator-activated receptor-gamma coactivator-1alpha signaling is redox sensitive. Free Radic Biol Med. 2009;47(10):1394-400. https://pubmed.ncbi.nlm.nih.gov/19686839. https://doi.org/10.1016/j.freeradbiomed.2009.08.007.
Cardaci S, Filomeni G, Ciriolo MR. Redox implications of AMPK-mediated signal transduction beyond energetic clues. J Cell Sci 2012;125(Pt 9):2115-25. https://pubmed.ncbi.nlm.nih.gov/22619229. https://doi.org/10.1242/jcs.095216.
Puigserver P, Spiegelman BM. Peroxisome proliferator-activated receptor-gamma coactivator 1 alpha (PGC-1 alpha): Transcriptional coactivator and metabolic regulator. Endocr Rev. 2003;24(1):78-90. https://pubmed.ncbi.nlm.nih.gov/12588810. https://doi.org/10.1210/er.2002-0012.
Wu Z, Puigserver P, Andersson U, et al. Mechanisms controlling mitochondrial biogenesis and respiration through the thermogenic coactivator PGC-1. Cell 1999;98(1):115-24. https://pubmed.ncbi.nlm.nih.gov/10412986. https://doi.org/10.1016/S0092-8674(00)80611-X.
Vargas-Mendoza N, Morales-Gonzalez A, Madrigal-Santillan EO, et al. Antioxidant and adaptative response mediated by Nrf2 during physical exercise. Antioxidants (Basel). 2019;8(6): E196. https://pubmed.ncbi.nlm.nih.gov/31242588. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6617290. https://doi.org/10.3390/antiox8060196.
Burk RF, Hill KE. Selenoprotein P-expression, functions, and roles in mammals. BBA 2009;1790(11):1441-7. https://pubmed.ncbi.nlm.nih.gov/19345254. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2763998. https://doi.org/10.1016/j.bbagen.2009.03.026.
Saito Y, Sato N, Hirashima M, Takebe G, Nagasawa S, Takahashi K. Domain structure of bi-functional selenoprotein P. Biochem J. 2004;381(Pt 3):841-6. https://pubmed.ncbi.nlm.nih.gov/15117283. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1133894. https://doi.org/10.1042/BJ20040328.
Misu H, Takayama H, Saito Y, et al. Deficiency of the hepatokine selenoprotein P increases responsiveness to exercise in mice through upregulation of reactive oxygen species and AMP-activated protein kinase in muscle. Nat Med. 2017;23(4):508-16. https://pubmed.ncbi.nlm.nih.gov/28263310. https://doi.org/10.1038/nm.4925.
Misu H, Takamura T, Takayama H, et al. A liver-derived secretory protein, selenoprotein P, causes insulin resistance. Cell Metab. 2010;12(5):483-95. https://pubmed.ncbi.nlm.nih.gov/21035759. https://doi.org/10.1016/j.cmet.2010.09.015.
Misu H, Ishikura K, Kurita S, et al. Inverse correlation between serum levels of selenoprotein P and adiponectin in patients with type 2 diabetes. PloS One. 2012;7(4):e34952. https://pubmed.ncbi.nlm.nih.gov/22496878. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3319626. https://doi.org/10.1371/journal.pone.0034952.
Ishikura K, Misu H, Kumazaki M, et al. Selenoprotein P as a diabetes-associated hepatokine that impairs angiogenesis by inducing VEGF resistance in vascular endothelial cells. Diabetologia. 2014;57(9):1968-76. https://pubmed.ncbi.nlm.nih.gov/24989996. https://doi.org/10.1007/s00125-014-3306-9.
Clay Montier LL, Deng JJ, Bai Y. Number matters: Control of mammalian mitochondrial DNA copy number. J Genet Genomics. 2009;36(3):125-31. https://pubmed.ncbi.nlm.nih.gov/19302968. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4706993. https://doi.org/10.1016/S1673-8527(08)60099-5.
Maynar M, Llerena F, Bartolomé I, et al. Seric concentrations of copper, chromium, manganese, nickel, and selenium in aerobic, anaerobic, and mixed professional sportsmen. J Int Soc Sports Nutr. 2018;15:8. https://pubmed.ncbi.nlm.nih.gov/29449792. https://doi.org/10.1186/s12970-018-0212-4.
Oo SM, Misu H, Saito Y, et al. Serum selenoprotein P, but not selenium, predicts future hyperglycemia in a general Japanese population. Sci Rep. 2018;8(1):16727. https://pubmed.ncbi.nlm.nih.gov/30425271. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6233151. https://doi.org/10.1038/s41598-018-35067-2.
Bernard RR. Fundamentals of Biostatistics, 5th ed. USA: Duxbury Press; 2000.
Centers for disease control and prevention. Physical activity guidelines for Americans. Available at https://health.gov/sites/default/files/2019-09/ Physical_Activity_Guidelines_2nd_edition.pdf. Accessed May 30, 2021.
Chang YK, Kim DE, Cho SH, Kim JH. Association between leukocyte mitochondrial DNA copy number and regular exercise in postmenopausal women. Korean J Fam Med. 2016;37(6):334-9. https://pubmed.ncbi.nlm.nih.gov/27900071. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5122665. https://doi.org/10.4082/kjfm.2016.37.6.334.
Misu H, Takamura T. Regulation of glucose metabolism by liver-derived secretory proteins 'hepatokines'. Nihon Rinsho. 2012;70 Suppl 3:207-11. https://pubmed.ncbi.nlm.nih.gov/22768521.
Mita Y, Nakayama K, Inari S, et al. Selenoprotein P-neutralizing antibodies improve insulin secretion and glucose sensitivity in type 2 diabetes mouse models. Nat Commun. 2017;8(1):1658. https://pubmed.ncbi.nlm.nih.gov/29162828. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5698464. https://doi.org/10.1038/s41467-017-01863-z.
Motahari-Tabari N, Ahmad Shirvani M, Shirzad-E-Ahoodashty M, Yousefi-Abdolmaleki E, Teimourzadeh M. The effect of 8 weeks aerobic exercise on insulin resistance in type 2 diabetes: A randomized clinical trial. Glob J Health Sci 2014;7(1):115-21. https://pubmed.ncbi.nlm.nih.gov/25560330. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4796439. https://doi.org/10.5539/gjhs.v7n1p115.
Jelleyman C, Yates T, O'Donovan G, et al. The effects of high-intensity interval training on glucose regulation and insulin resistance: A meta-analysis. Obes Rev. 2015;16(11):942-61. https://pubmed.ncbi.nlm.nih.gov/26481101. https://doi.org/10.1111/obr.12317.
Winding KM, Munch GW, Iepsem UW, Hall GV, Pederson BK, Mortensen SP. The effect on glycemic control of low-volume high-intensity interval training in individuals with type 2 diabetes. Diabetes Obes Metab. 2018;20(5):1131-9. https://pubmed.ncbi.nlm.nih.gov/29272072. https://doi.org/10.1111/dom.13198.
Sargeant JA, Aithal GP, Takamura T, et al. The influence of adiposity and acute exercise on circulating hepatokines in normal-weight and overweight/obese men. Appl Physiol Nutr Metab. 2018;43(5):482-90. https://pubmed.ncbi.nlm.nih.gov/29220580. https://doi.org/10.1139/apnm-2017-0639.
Kikuchi N, Satoh K, Kurosawa R, et al. Selenoprotein P promotes the development of pulmonary arterial hypertension: Possible novel therapeutic target. Circulation. 2018:138(6):600-23. https://pubmed.ncbi.nlm.nih.gov/29636330. https://doi.org/10.1161/CIRCULATIONAHA. 117.033113.
Yang SJ, Hwang SY, Choi HY, et al. Serum selenoprotein P levels in patients with type 2 diabetes and prediabetes: Implications for insulin resistance, inflammation, and atherosclerosis. J Clin Endocrinol Metab. 2011;96(8):E1325-9. https://pubmed.ncbi.nlm.nih.gov/21677040. https://doi.org/10.1210/jc.2011-0620.
Strauss E, Tomczak J, Staniszewski R, Oszkinis G. Associations and interactions between variants in selenoprotein genes, selenoprotein levels and the development of abdominal aortic aneurysm, peripheral arterial disease, and heart failure. PLoS One. 2018;13(9):e0203350. https://pubmed.ncbi.nlm.nih.gov/30188935. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6126836. https://doi.org/10.1371/journal.pone.0203350.
Schweizer U, Streckfuss F, Pelt P, et al. Hepatically derived selenoprotein P is a key factor for kidney but not for brain selenium supply. Biochem J. 2005;386(Pt 2):221-6. https://pubmed.ncbi.nlm.nih.gov/15638810. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1134785. https://doi.org/10.1042/BJ20041973.
Takayama H, Misu H, Iwama H, et al. Metformin suppresses expression of the selenoprotein P gene via an AMP-activated kinase (AMPK)/FoxO3a pathway in H4IIEC3 hepatocytes. J Biol Chem. 2014;289(1):335-45. https://pubmed.ncbi.nlm.nih.gov/24257750. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3879556. https://doi.org/10.1074/jbc.M113.479386.
Ruderman NB, Park H, Kaushik VK, et al. AMPK as a metabolic switch in rat muscle, liver, and adipose tissue after exercise. Acta Physiol Scand. 2003;178(4):435-42. https://pubmed.ncbi.nlm.nih.gov/12864749. https://doi.org/10.1046/j.1365-201X.2003.01164.x.
Choi SL, Kim SJ, Lee KT, et al. The regulation of AMP-activated protein kinase by H(2)O(2). Biochem Biophys Res Commun 2001;287(1):92-7. https://pubmed.ncbi.nlm.nih.gov/11549258. https://doi.org/10.1006/bbrc.2001.5544.
Ji LL. Antioxidant signaling in skeletal muscle: A brief review. Exp Gerontol. 2007;42(7):582-93. https://pubmed.ncbi.nlm.nih.gov/17467943. https://doi.org/10.1016/j.exger.2007.03.002.
Powers SK, Jackson MJ. Exercise-induced oxidative stress: Cellular mechanisms and impact on muscle force production. Physiol Rev. 2008;88(4):1243-76. https://pubmed.ncbi.nlm.nih.gov/18923182. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2909187. https://doi.org/10.1152/physrev.00031.2007.
Timmerman KL, Ballard KD, Deal MA, et al. Associations among physical activity level and skeletal muscle antioxidants in older adults. J Phys Act Health. 2020;17(9):895-901. https://pubmed.ncbi.nlm.nih.gov/32788413. https://doi.org/10.1123/jpah.2020-0082.
Irrcher I, Ljubicic V, Hood DA. Interactions between ROS and AMP kinase activity in the regulation of PGC-1alpha transcription in skeletal muscle cells. Am J Physiol Cell Physiol. 2009;296(1): C116-23. https://pubmed.ncbi.nlm.nih.gov/19005163. https://doi.org/10.1152/ajpcell.00267.2007.
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2023 Swe Mar Oo, Min Thar Htut, Ye Win Htun, Aye Aye Mon, May Pyone Kyaw

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
The full license is at this link: http://creativecommons.org/licenses/by-nc/3.0/legalcode).
To obtain permission to translate/reproduce or download articles or use images FOR COMMERCIAL REUSE/BUSINESS PURPOSES from the Journal of the ASEAN Federation of Endocrine Societies, kindly fill in the Permission Request for Use of Copyrighted Material and return as PDF file to jafes@asia.com or jafes.editor@gmail.com.
A written agreement shall be emailed to the requester should permission be granted.










