The Effect of Glucocorticoids on TAF1 Gene Transcription in X-linked Dystonia Parkinsonism
DOI:
https://doi.org/10.15605/jafes.037.S6Keywords:
XDP, glucocorticoids, stress, neurodegenerative disease, TAF1Abstract
Objective. X-linked Dystonia Parkinsonism (XDP) is associated with a SINE-VNTR- Alu (SVA) retrotransposon insertion in an intron of the TAF1 gene that alters gene transcription and splicing. In this study, we determined if the SVA insertion introduces glucocorticoid (GC)-responsive cis-regulatory elements that may contribute to dysregulated TAF1 transcription and XDP disease progression.
Methodology. We performed in silico analysis to identify potential GC receptor (GR) binding sites within the XDP-SVA. We also conducted promoter-reporter assays on HeLa and HEK293T cells to assess the intrinsic promoter activity of three XDP-SVA variants representing different hexameric repeat lengths associated with differences in disease onset. We treated XDP fibroblast cell models with GR agonist (CORT) or antagonist (RU486), then subjected TAF1 and the XDP-associated aberrant transcript, TAF1-32i to gene expression analysis.
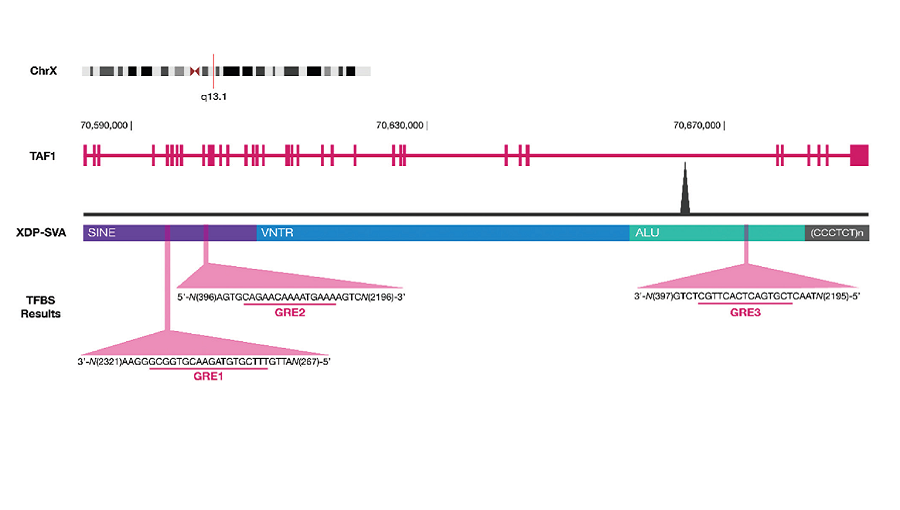
Results. A transcription factor binding site search revealed three binding sites for GR within the XDP-SVA—two within the SINE region and one in the Alu region. Promoter-reporter assays showed induction of XDP-SVA promoter activity upon CORT treatment that was dependent on the cell line and XDP-SVA hexamer repeat length. Gene expression analysis showed that baseline TAF1 levels differed between control and patient fibroblast cell lines, and treatment with CORT led to an increasing trend in the expression of the aberrant TAF1-32i transcript but did not reach statistical significance. Treatment with RU486 increased TAF1 mRNA expression only in the control cell lines.
Conclusion. Using reporter assays, the XDP-SVA was shown to exhibit CORT-dependent transcriptional activation. Gene expression analysis also showed that GC signaling may influence TAF1 and TAF1-32i expression, possibly through interaction with the XDP-SVA. Our data provide a potential link between stress and XDP progression.
Downloads
References
Rosales RL. X-linked dystonia parkinsonism: Clinical phenotype, genetics and therapeutics. J Mov Disord. 2010;3(2):32-8. https://pubmed.ncbi.nlm.nih.gov/24868378. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4027667. https://doi.org/10.14802/jmd.10009.
Diestro JDB, Pasco PMD, Lee LV. Validation of a screening questionnaire for X-linked dystonia parkinsonism: The first phase of the population-based prevalence study of X-linked dystonia parkinsonism in Panay. Neurol Clin Neurosci. 2017;5(3):79-85. https://doi.org/10.1111/ncn3.12113.
Lee LV, Rivera C, Teleg RA, et al. The unique phenomenology of sex-linked dystonia parkinsonism (XDP, DYT3, "Lubag"). Int J Neurosci. 2011;121 Suppl 1:3-11. https://pubmed.ncbi.nlm.nih.gov/21047175. https://doi.org/ 10.3109/00207454.2010.526728.
Evidente VGH, Nolte D, Niemann S, et al. Phenotypic and molecular analyses of X-linked dystonia-parkinsonism (“lubag”) in women. Archives of Neurology. 2004;61(12):1956-9. https://pubmed.ncbi.nlm.nih.gov/15596620. https://doi.org/10.1001/archneur.61.12.1956.
Aneichyk T, Hendriks WT, Yadav R, et al. Dissecting the causal mechanism of X-linked dystonia-parkinsonism by integrating genome and transcriptome assembly. Cell. 2018;172(5):897-909.e21. https://pubmed.ncbi.nlm.nih.gov/29474918. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5831509. https://doi.org/10.1016/j.cell.2018.02.011.
Bragg DC, Mangkalaphiban K, Vaine CA, et al. Disease onset in X-linked dystonia-parkinsonism correlates with expansion of a hexameric repeat within an SVA retrotransposon in TAF1. Proc Natl Acad Sci U S A. 2017;114(51):E11020-8. https://pubmed.ncbi.nlm.nih.gov/29229810. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5754783. https://doi.org/10.1073/pnas.1712526114.
Pozojevic J, Algodon SM, Cruz JN, et al. Transcriptional alterations in X-linked dystonia parkinsonism caused by the SVA retrotransposon. Int J Mol Sci. 2022;23(4):2231. https://pubmed.ncbi.nlm.nih.gov/35216353. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8875906. https://doi.org/10.3390/ijms23042231.
Alcachupas A, Bellosillo K, Catolico WR, et al. Biopsychosocial aspect of patients with X-linked dystonia-parkinsonism: Its implications on quality of life. Cureus. 2022;14(1):e21699. https://pubmed.ncbi.nlm.nih.gov/35242471. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8884542. https://doi.org/10.7759/cureus.21699.
Morigaki R, Nakataki M, Kawarai T, et al. Depression in X-linked dystonia-parkinsonism: A case-control study. Parkinsonism Relat Disord. 2013;19(9):844-6. https://pubmed.ncbi.nlm.nih.gov/23706616. https://doi.org/10.1016/j.parkreldis.2013.04.027.
Jamora RD, Rae F. Cenina A, A. Teleg R, V. Lee L. Suicidality among patients with sex-linked dystonia-parkinsonism (XDP). Acta Medica Philipp. 2015;49(1):20-3. https://doi.org/10.47895/amp.v49i1.1009.
Heitzer MD, Wolf IM, Sanchez ER, Witchel SF, DeFranco DB. Glucocorticoid receptor physiology. Rev Endocr Metab Disord. 2007;8(4):321-30. https://pubmed.ncbi.nlm.nih.gov/18049904. https://doi.org/10.1007/s11154-007-9059-8.
Timmermans S, Souffriau J, Libert C. A general introduction to glucocorticoid biology. Front Immunol. 2019;10:1545. https://pubmed.ncbi.nlm.nih.gov/31333672. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6621919. https://doi.org/10.3389/fimmu.2019.01545.
Tost H, Champagne FA, Meyer-Lindenberg A. Environmental influence in the brain, human welfare and mental health. Nat Neurosci. 2015;18(10):1421-31. https://pubmed.ncbi.nlm.nih.gov/26404717. https://doi.org/10.1038/nn.4108.
Bagamasbad P, Ziera T, Borden SA, et al. Molecular basis for glucocorticoid induction of the Kruppel-like factor 9 gene in hippocampal neurons. Endocrinology. 2012;153(11):5334-45. https://pubmed.ncbi.nlm.nih.gov/22962255. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3473204. https://doi.org/10.1210/en.2012-1303.
Herrero M-T, Estrada C, Maatouk L, Vyas S. Inflammation in Parkinson's disease: Role of glucocorticoids. Frontiers in neuroanatomy. 2015;9:32. https://pubmed.ncbi.nlm.nih.gov/25883554. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4382972. https://doi.org/10.3389/fnana.2015.00032.
Okawa Y, Ishiguro K, Fujita SC. Stress‐induced hyperphosphorylation of tau in the mouse brain. FEBS letters. 2003;535(1-3):183-9. https://pubmed.ncbi.nlm.nih.gov/12560101. https://doi.org/10.1016/s0014-5793(02)03883-8.
Lee C, Huang CH. LASAGNA-Search 2.0: Integrated transcription factor binding site search and visualization in a browser. Bioinformatics. 2014;30(13):1923-5. https://pubmed.ncbi.nlm.nih.gov/24578403. https://doi.org/10.1093/bioinformatics/btu115.
Jacobsen BM, Jambal P, Schittone SA, Horwitz KB. ALU repeats in promoters are position-dependent co-response elements (coRE) that enhance or repress transcription by dimeric and monomeric progesterone receptors. Mol Endocrinol. 2009;23(7):989-1000. https://pubmed.ncbi.nlm.nih.gov/19372234. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2703596. https://doi.org/10.1210/me.2009-0048.
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2023 Sam Ezrael Dela Cruz, Pia Bagamasbad

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
The full license is at this link: http://creativecommons.org/licenses/by-nc/3.0/legalcode).
To obtain permission to translate/reproduce or download articles or use images FOR COMMERCIAL REUSE/BUSINESS PURPOSES from the Journal of the ASEAN Federation of Endocrine Societies, kindly fill in the Permission Request for Use of Copyrighted Material and return as PDF file to jafes@asia.com or jafes.editor@gmail.com.
A written agreement shall be emailed to the requester should permission be granted.










