Review of Literature on Akkermansia muciniphila and its Possible Role in the Etiopathogenesis and Therapy of Type 2 Diabetes Mellitus
DOI:
https://doi.org/10.15605/jafes.037.01.13Keywords:
Akkermansia muciniphila, ER stress, gut microbiota, insulin resistance, probiotic, type 2 diabetes mellitusAbstract
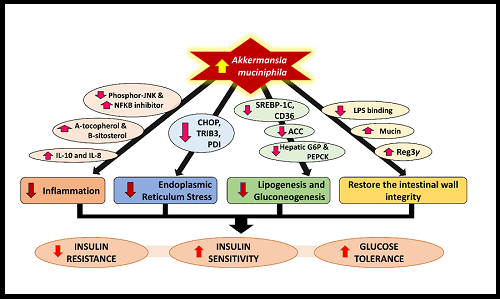
Akkermansia muciniphila is a promising gut microbiota for the treatment of type 2 diabetes mellitus (T2DM). A. muciniphila stimulates intestinal wall integrity, is an anti-inflammatory agent, and reduces endoplasmic reticulum stress, lipogenesis and gluconeogenesis. These properties make A. muciniphila a potential treatment option for T2DM by reducing insulin resistance and increasing insulin sensitivity and glucose tolerance in different tissues. This article explores the possible role of A. muciniphila in T2DM management, along with the various methods known to modulate A. muciniphila.
Downloads
References
Carding S, Verbeke K, Vipond DT, Corfe BM, Owen LJ. Dysbiosis of the gut microbiota in disease. Microb Ecol Health Dis. 2015;26:26191. https://pubmed.ncbi.nlm.nih.gov/25651997. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4315779. https://doi.org/10.3402/mehd.v26.26191.
Wen L, Duffy A. Factors influencing the gut microbiota, inflammation, and type 2 diabetes. J Nutr. 2017;147(7):1468S-75S. https://pubmed.ncbi.nlm.nih.gov/28615382. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5483960. https://doi.org/10.3945/jn.116.240754.
Kim YA, Keogh JB, Clifton PM. Probiotics, prebiotics, synbiotics and insulin sensitivity. Nutr Res Rev. 2018;31(1):35-51. https://pubmed.ncbi.nlm.nih.gov/29037268. https://doi.org/10.1017/S095442241700018X.
Collado MC, Derrien M, Isolauri E, de Vos WM, Salminen S. Intestinal integrity and Akkermansia muciniphila, a mucin-degrading member of the intestinal microbiota present in infants, adults, and the elderly. Appl Environ Microbiol. 2007;73(23):7767-70. https://pubmed.ncbi.nlm.nih.gov/17933936. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2168041. https://doi.org/10.1128/AEM.01477-07.
Rosenbaum M, Knight R, Leibel RL. The gut microbiota in human energy homeostasis and obesity. Trends Endocrinol Metabol. 2015;26(9):493-501. https://pubmed.ncbi.nlm.nih.gov/26257300. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4862197. https://doi.org/10.1016/j.tem.2015.07.002.
Yabe D, Kuwata H, Seino Y. The journey to understanding incretin systems: Theory, practice and more theory. J Diabetes Investig. 2019;10(5):1171-3. https://pubmed.ncbi.nlm.nih.gov/31361402. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6717807. https://doi.org/10.1111/jdi.13123.
Han JL, Lin HL. Intestinal microbiota and type 2 diabetes: From mechanism insights to therapeutic perspective. World J Gastroenterol. 2014;20(47):17737-45. https://pubmed.ncbi.nlm.nih.gov/25548472. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4273124. https://doi.org/10.3748/wjg.v20.i47.17737.
Zhao S, Liu W, Wang J, et al. Akkermansia muciniphila improves metabolic profiles by reducing inflammation in chow diet-fed mice. J Mol Endocrinol. 2017;58(1):1-14. https://pubmed.ncbi.nlm.nih.gov/27821438. https://doi.org/10.1530/JME-16-0054.
Pero R, Fico G, Scudiero O, Laneri S. Microbiota and LPS-induced obesity inflammation: Therapeutic implications. Preprints. 2018:2018070375. https:// 10.20944/preprints201807.0375.v1.
Belzer C, De Vos WM. Microbes inside—from diversity to function: The case of Akkermansia. ISME J. 2012;6(8):1449-58. https://pubmed.ncbi.nlm.nih.gov/22437156. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3401025. https://doi.org/10.1038/ismej.2012.6.
Derrien M, Vaughan EE, Plugge CM, de Vos WM. Akkermansia muciniphila gen. nov., sp. nov., a human intestinal mucin-degrading bacterium. Int J Syst Evol Microbiol. 2004;54(Pt 5):1469-76. https://pubmed.ncbi.nlm.nih.gov/15388697. https://doi.org/10.1099/ijs.0.02873-0.
Karlsson CL, Önnerfält J, Xu J, Molin G, Ahrné S, Thorngren‐Jerneck K. The microbiota of the gut in preschool children with normal and excessive body weight. Obesity (Silver Spring). 2012;20(11):2257-61. https://pubmed.ncbi.nlm.nih.gov/22546742. https://doi.org/10.1038/oby.2012.110.
Dao MC, Everard A, Aron-Wisnewsky J, et al. Akkermansia muciniphila and improved metabolic health during a dietary intervention in obesity: Relationship with gut microbiome richness and ecology. Gut. 2016;65(3):426-36. https://pubmed.ncbi.nlm.nih.gov/26100928. https://doi.org/10.1136/gutjnl-2014-308778.
Schneeberger M, Everard A, Gómez-Valadés AG, et al. Akkermansia muciniphila inversely correlates with the onset of inflammation, altered adipose tissue metabolism and metabolic disorders during obesity in mice. Sci Rep. 2015;5:16643. https://pubmed.ncbi.nlm.nih.gov/26563823. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4643218. https://doi.org/10.1038/srep16643.
Romaní J, Caixàs A, Escoté X, et al. Lipopolysaccharide‐binding protein is increased in patients with psoriasis with metabolic syndrome, and correlates with C‐reactive protein. Clin Exp Dermatol. 2013;38(1):81-4. https://pubmed.ncbi.nlm.nih.gov/23082944. https://doi.org/10.1111/ced.12007.
Tsaousidou E, Paeger L, Belgardt BF, et al. Distinct roles for JNK and IKK activation in agouti-related peptide neurons in the development of obesity and insulin resistance. Cell Rep. 2014;9(4):1495-506. https://pubmed.ncbi.nlm.nih.gov/25456138. https://doi.org/10.1016/j.celrep.2014.10.045.
Greer RL, Dong X, Moraes ACF, et al. Akkermansia muciniphila mediates negative effects of IFNγ on glucose metabolism. Nat Commun. 2016;7:13329. https://pubmed.ncbi.nlm.nih.gov/27841267. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5114536. https://doi.org/10.1038/ncomms13329.
Loizou S, Lekakis I, Chrousos GP, Moutsatsou P. β‐Sitosterol exhibits anti‐inflammatory activity in human aortic endothelial cells. Mol Nutr Food Res. 2010;54(4):551-8. https://pubmed.ncbi.nlm.nih.gov/19937850. https://doi.org/10.1002/mnfr.200900012.
Remely M, Hippe B, Zanner J, Aumueller E, Brath H, G Haslberger A. Gut microbiota of obese, type 2 diabetic individuals is enriched in Faecalibacterium prausnitzii, Akkermansia muciniphila and Peptostreptococcus anaerobius after weight loss. Endocr Metab Immune Disord Drug Targets. 2016;16(2):99-106. https://pubmed.ncbi.nlm.nih.gov/27577947. https://doi.org/10.2174/1871530316666160831093813.
Cnop M, Foufelle F, Velloso LA. Endoplasmic reticulum stress, obesity and diabetes. Trends Mol Med. 2012;18(1):59-68. https://pubmed.ncbi.nlm.nih.gov/21889406. https://doi.org/10.1016/j.molmed.2011.07.010.
Lochhead PA, Salt IP, Walker KS, Hardie DG, Sutherland C. 5-aminoimidazole-4-carboxamide riboside mimics the effects of insulin on the expression of the 2 key gluconeogenic genes PEPCK and glucose-6-phosphatase. Diabetes. 2000;49(6):896-903. https://pubmed.ncbi.nlm.nih.gov/10866040. https://doi.org/10.2337/diabetes.49.6.896.
Marcial-Coba MS, Cieplak T, Cahú TB, Blennow A, Knøchel S, Nielsen DS. Viability of microencapsulated Akkermansia muciniphila and Lactobacillus plantarum during freeze-drying, storage and in vitro simulated upper gastrointestinal tract passage. Food Funct. 2018;9(11):5868-79. https://pubmed.ncbi.nlm.nih.gov/30362482. https://doi.org/10.1039/c8fo01331d.
Ropot AV, Karamzin AM, Sergeyev OV. Cultivation of the next-generation probiotic Akkermansia muciniphila, methods of its safe delivery to the intestine, and factors contributing to its growth in vivo. Curr Microbiol. 2020;77(8):1363-72. https://pubmed.ncbi.nlm.nih.gov/32318863. https://doi.org/10.1007/s00284-020-01992.
Depommier C, Everard A, Druart C, et al. Supplementation with Akkermansia muciniphila in overweight and obese human volunteers: A proof-of-concept exploratory study. Nat Med. 2019;25(7):1096-103. https://pubmed.ncbi.nlm.nih.gov/31263284. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6699990. https://doi.org/10.1038/s41591-019-0495-2.
Everard A, Lazarevic V, Derrien M, et al. Responses of gut microbiota and glucose and lipid metabolism to prebiotics in genetic obese and diet-induced leptin-resistant mice. Diabetes. 2011;60(11):2775-86. https://pubmed.ncbi.nlm.nih.gov/21933985. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3198091. https://doi.org/10.2337/db11-0227.
Roopchand DE, Carmody RN, Kuhn P, et al. Dietary polyphenols promote growth of the gut bacterium Akkermansia muciniphila and attenuate high-fat diet–induced metabolic syndrome. Diabetes. 2015;64(8):2847-58. https://pubmed.ncbi.nlm.nih.gov/25845659. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4512228. https://doi.org/10.2337/db14-1916.
Tu P, Bian X, Chi L, Gao B, Ru H, Knobloch TJ, et al. Characterization of the functional changes in mouse gut microbiome associated with increased Akkermansia muciniphila population modulated by dietary black raspberries. ACS omega. 2018;3(9):10927-37. https://pubmed.ncbi.nlm.nih.gov/25845659. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4512228. https://doi.org/10.2337/db14-1916.
Masumoto S, Terao A, Yamamoto Y, Mukai T, Miura T, Shoji T. Non-absorbable apple procyanidins prevent obesity associated with gut microbial and metabolomic changes. Sci Rep. 2016;6:31208. https://pubmed.ncbi.nlm.nih.gov/27506289. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4979010. https://doi.org/10.1038/srep31208.
Forslund K, Hildebrand F, Nielsen T, et al. Disentangling type 2 diabetes and metformin treatment signatures in the human gut microbiota. Nature. 2015;528(7581):262-6. https://pubmed.ncbi.nlm.nih.gov/26633628. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4681099. https://doi.org/10.1038/nature15766.
Shin NR, Lee JC, Lee HY, et al. An increase in the Akkermansia spp. population induced by metformin treatment improves glucose homeostasis in diet-induced obese mice. Gut. 2014;63(5):727-35. https://pubmed.ncbi.nlm.nih.gov/23804561. https://doi.org/10.1136/gutjnl-2012-303839.
De La Cuesta-Zuluaga J, Mueller NT, Corrales-Agudelo V, et al. Metformin is associated with higher relative abundance of mucin-degrading Akkermansia muciniphila and several short-chain fatty acid–producing microbiota in the gut. Diabetes Care. 2017;40(1):54-62. https://pubmed.ncbi.nlm.nih.gov/27999002. https://doi.org/10.2337/dc16-1324.
Ulker I, Yildiran H. The effects of bariatric surgery on gut microbiota in patients with obesity: A review of the literature. Biosci Microbiota, Food Health. 2019;38(1):3-9. https://pubmed.ncbi.nlm.nih.gov/30705797. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6343052. https://doi.org/10.12938/bmfh.18-018.
Murphy R, Tsai P, Jüllig M, Liu A, Plank L, Booth M. Differential changes in gut microbiota after gastric bypass and sleeve gastrectomy bariatric surgery vary according to diabetes remission. Obes Surg. 2017;27(4):917-25. https://pubmed.ncbi.nlm.nih.gov/27738970. https://doi.org/10.1007/s11695-016-2399-2.
Debédat J, Clément K, Aron-Wisnewsky J. Gut microbiota dysbiosis in human obesity: Impact of bariatric surgery. Curr Obes Rep. 2019;8(3):229-42. https://pubmed.ncbi.nlm.nih.gov/31197613. https://doi.org/10.1007/s13679-019-00351-3.
Cani PD. Severe obesity and gut microbiota: Does bariatric surgery really reset the system? Gut. 2019;68(1):5-6. https://pubmed.ncbi.nlm.nih.gov/29991642. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6839830. https://doi.org/10.1136/gutjnl-2018-316815.
Queipo-Ortuño MI, Seoane LM, Murri M, et al. Gut microbiota composition in male rat models under different nutritional status and physical activity and its association with serum leptin and ghrelin levels. PloS One. 2013;8(5):e65465. https://pubmed.ncbi.nlm.nih.gov/23724144. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3665787. https://doi.org/10.1371/journal.pone.0065465.
Dao MC, Belda E, Prifti E, et al. Akkermansia muciniphila abundance is lower in severe obesity, but its increased level after bariatric surgery is not associated with metabolic health improvement. Am J Physiol Endocrinol Metab. 2019;317(3):E446-59. https://pubmed.ncbi.nlm.nih.gov/31265324. https://doi.org/10.1152/ajpendo.00140.2019.
Costello SP, Tucker EC, La Brooy J, Schoeman MN, Andrews JM. Establishing a fecal microbiota transplant service for the treatment of Clostridium difficile infection. Clin Infect Dis. 2016;62(7):908-14. https://pubmed.ncbi.nlm.nih.gov/26628567. https://doi.org/10.1093/cid/civ994.
Sunkara T, Rawla P, Ofosu A, Gaduputi V. Fecal microbiota transplant–A new frontier in inflammatory bowel disease. J Inflamm Res. 2018;11:321-8. https://pubmed.ncbi.nlm.nih.gov/30214266. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6124474. https://doi.org/10.2147/JIR.S176190.
van Reenen CA, Dicks LM. Horizontal gene transfer amongst probiotic lactic acid bacteria and other intestinal microbiota: What are the possibilities? A review. Arch Microbiol. 2011;193(3):157-68. https://pubmed.ncbi.nlm.nih.gov/21193902. https://doi.org/10.1007/s00203-010-0668-3.
Nawaz M, Wang J, Zhou A, et al. Characterization and transfer of antibiotic resistance in lactic acid bacteria from fermented food products. Curr Microbiol. 2011;62(3):1081-9. https://pubmed.ncbi.nlm.nih.gov/21212956. https://doi.org/10.1007/s00284-010-9856-2.
Zheng M, Zhang R, Tian X, Zhou X, Pan X, Wong A. Assessing the risk of probiotic dietary supplements in the context of antibiotic resistance. Front Microbiol. 2017;8:908. https://pubmed.ncbi.nlm.nih.gov/28579981. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5437161. https://doi.org/10.3389/fmicb.2017.00908.
Published
How to Cite
Issue
Section
License
Journal of the ASEAN Federation of Endocrine Societies is licensed under a Creative Commons Attribution-NonCommercial 4.0 International. (full license at this link: http://creativecommons.org/licenses/by-nc/3.0/legalcode).
To obtain permission to translate/reproduce or download articles or use images FOR COMMERCIAL REUSE/BUSINESS PURPOSES from the Journal of the ASEAN Federation of Endocrine Societies, kindly fill in the Permission Request for Use of Copyrighted Material and return as PDF file to jafes@asia.com or jafes.editor@gmail.com.
A written agreement shall be emailed to the requester should permission be granted.









