Prevalence of Bacterial Urinary Tract Infection Among Patients With Type 2 Diabetes Mellitus on Sodium-Glucose Cotransporter-2 Inhibitors
A Prospective Real-World Setting Study
DOI:
https://doi.org/10.15605/jafes.037.02.04Keywords:
SGLT2i, type 2 diabetes mellitus, UTI, significant bacteriuriaAbstract
Background. Genitourinary tract infections, mycotic as well as bacterial, as defined by clinical symptoms, are one of the common adverse effects associated with the use of sodium-glucose cotransporter-2 inhibitors (SGLT2i) in type 2 diabetes mellitus (T2DM) patients in clinical trials. However, Indian data in terms of the prevalence of culture-proven bacterial type of urinary tract infection (UTI), and the causative organism is limited.
Objective. This study aimed to determine the prevalence and causative agents of bacterial UTI among patients with T2DM on SGLT2i.
Methodology. This was a prospective longitudinal study involving all patients with T2DM who were prescribed with SGLT2i, uncontrolled on other oral anti-diabetic medications, from June 2019 to February 2020. Prevalence of bacterial UTI was evaluated at baseline and 12 weeks after initiation of SGLT2i.
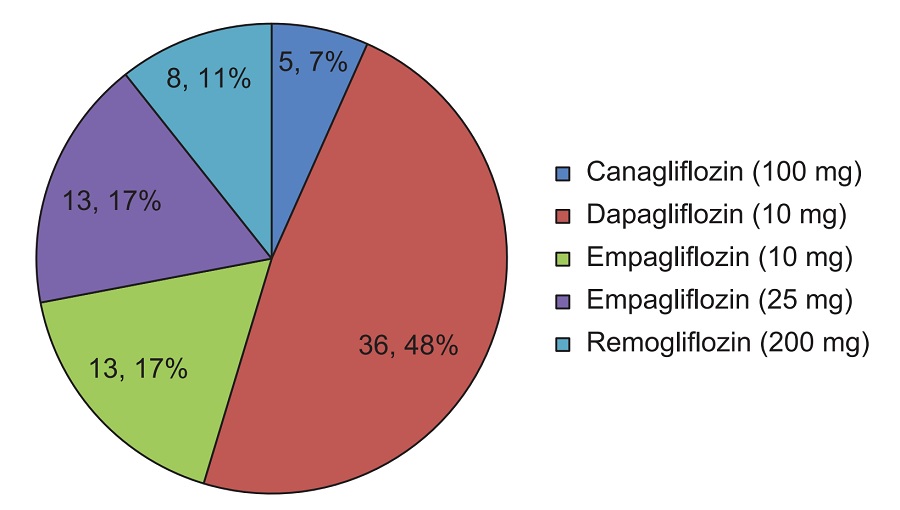
Results. A total of 80 patients were started on SGLT2i. One female patient on canagliflozin had significant asymptomatic bacteriuria and the causative agent was Acinetobacter baumannii. One male patient on dapagliflozin had symptomatic urinary tract infection with negative urine culture study. Four patients developed genital mycotic infection.
Conclusion. In this real-world study, SGLT2i as a class, was well tolerated with favorable safety profile, and risk of developing significant bacteriuria and/or symptomatic UTI was minimal.
Downloads
References
Kaveeshwar SA, Cornwall J. The current state of diabetes mellitus in India. Australas Med J. 2014;7(1):45-8. https://pubmed.ncbi.nlm.nih.gov/24567766. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3920109. https://doi.org/10.4066/AMJ.2013.1979.
Yesudian CA, Grepstad M, Visintin E, Ferrario A. The economic burden of diabetes in India: A review of the literature. Global Health. 2014;10:80. https://pubmed.ncbi.nlm.nih.gov/25443136. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4279984. https://doi.org/10.1186/s12992-014-0080-x
George RE, Joseph S. A review of newer treatment approaches for type-2 diabetes: Focusing safety and efficacy of incretin based therapy. Saudi Pharm J. 2014;22(5):403-10. https://pubmed.ncbi.nlm.nih.gov/25473328. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4246366. https://doi.org/10.1016/j.jsps.2013.05.005.
Kalra S, Sahay R, Gupta Y. Sodium glucose transporter 2 (SGLT2) inhibition and ketogenesis. Indian J Endocrinol Metab. 2015 Jul-Aug;19(4):524-8. https://pubmed.ncbi.nlm.nih.gov/26180770. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4481661. https://doi.org/10.4103/2230-8210.157859.
Rabizadeh S, Nakhjavani M, Esteghamati A. Cardiovascular and renal benefits of SGLT2 inhibitors: A Narrative Review. Int J Endocrinol Metab. 2019;17(2):e84353. https://pubmed.ncbi.nlm.nih.gov/31372172. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6628616. https://doi.org/10.5812/ijem.84353.
Ribola FA, Cançado FB, Schoueri JH, De Toni VF, Medeiros VH, Feder D. Effects of SGLT2 inhibitors on weight loss in patients with type 2 diabetes mellitus. Eur Rev Med Pharmacol Sci. 2017;21(1):199-211. https://pubmed.ncbi.nlm.nih.gov/28121337.
Inzucchi SE, Bergenstal RM, Buse JB, et al. Management of hyperglycemia in type 2 diabetes: A patient-centered approach: Position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care. 2012;35(6):1364-79. https://pubmed.ncbi.nlm.nih.gov/22517736. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3357214. https://doi.org/10.2337/dc12-0413.
Zinman B, Wanner C, Lachin JM, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373(22):2117-28. https://pubmed.ncbi.nlm.nih.gov/26378978. https://doi.org/10.1056/NEJMoa1504720.
Nitzan O, Elias M, Chazan B, Saliba W. Urinary tract infections in patients with type 2 diabetes mellitus: review of prevalence, diagnosis, and management. Diabetes Metab Syndr Obes. 2015;8:129-36. https://pubmed.ncbi.nlm.nih.gov/25759592. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4346284. https://doi.org/10.2147/DMSO.S51792.
Liu J, Li L, Li S, Jia P, Deng K, Chen W, Sun X. Effects of SGLT2 inhibitors on UTIs and genital infections in type 2 diabetes mellitus: A systematic review and meta-analysis. Sci Rep. 2017;7(1):2824. https://pubmed.ncbi.nlm.nih.gov/28588220. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5460243. https://doi.org/10.1038/s41598-017-02733-w.
Hsia DS, Grove O, Cefalu WT. An update on sodium-glucose co-transporter-2 inhibitors for the treatment of diabetes mellitus. Curr Opin Endocrinol Diabetes Obes. 2017;24(1):73-79. https://pubmed.ncbi.nlm.nih.gov/27898586. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6028052. https://doi.org/10.1097/MED.0000000000000311.
Casqueiro J, Casqueiro J, Alves C. Infections in patients with diabetes mellitus: A review of pathogenesis. Indian J Endocrinol Metab. 2012;16 Suppl 1(Suppl1):S27-36. https://pubmed.ncbi.nlm.nih.gov/22701840. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3354930. https://doi.org/10.4103/2230-8210.94253.
Gill HK, Kaur P, Mahendru S, Mithal A. Adverse effect profile and effectiveness of sodium glucose co-transporter 2 inhibitors (SGLT2i) - A prospective real-world setting study. Indian J Endocrinol Metab. 2019;23(1):50-5. https://pubmed.ncbi.nlm.nih.gov/31016153. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6446693. https://doi.org/10.4103/ijem.IJEM_566_18.
Nicolle LE, Capuano G, Ways K, Usiskin K. Effect of canagliflozin, a sodium-glucose co-transporter 2 (SGLT2) inhibitor, on bacteriuria and urinary tract infection in subjects with type 2 diabetes enrolled in a 12-week, phase 2 study. Curr Med Res Opinion. 2012;28:1167-71. https://pubmed.ncbi.nlm.nih.gov/22548646. https://doi.org/10.1185/03007995.2012.689956.
Renko M, Tapanainen P, Tossavainen P, Pokka T, Uhari M. Meta-analysis of the significance of asymptomatic bacteriuria in diabetes. Diabetes Care. 2011;34(1):230-5. https://pubmed.ncbi.nlm.nih.gov/20937688. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3005460. https://doi.org/10.2337/dc10-0421.
List JF, Woo V, Morales E, Tang W, Fiedorek FT. Sodium-glucose cotransport inhibition with dapagliflozin in type 2 diabetes. Diabetes Care. 2009 Apr;32(4):650-7. https://pubmed.ncbi.nlm.nih.gov/19114612. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2660449. https://doi.org/10.2337/dc10-0421.
Bode B, Stenlöf K, Sullivan D, Fung A, Usiskin K. Efficacy and safety of canagliflozin treatment in older subjects with type 2 diabetes mellitus: A randomized trial. Hosp Pract (1995). 2013;41(2):72-84. https://pubmed.ncbi.nlm.nih.gov/23680739. https://doi.org/10.3810/hp.2013.04.1020.
Rosenstock J, Seman LJ, Jelaska A, Hantel S, Pinnetti S, Hach T, Woerle HJ. Efficacy and safety of empagliflozin, a sodium glucose cotransporter 2 (SGLT2) inhibitor, as add-on to metformin in type 2 diabetes with mild hyperglycaemia. Diabetes Obes Metab. 2013;15(12):1154-60. https://pubmed.ncbi.nlm.nih.gov/23906374. https://doi.org/10.1111/dom.12185.
Mathieu C, Ranetti AE, Li D, et al. Randomized, double-blind, phase 3 trial of triple therapy with dapagliflozin add-on to saxagliptin plus metformin in type 2 diabetes. Diabetes Care. 2015;38(11):2009-17. https://pubmed.ncbi.nlm.nih.gov/26246458. https://doi.org/10.2337/dc15-0779.
Sosale B, Sosale AR, Kumar PM, Joshi SR. A prospective analysis of the efficacy and safety of sodium glucose cotransporter 2 inhibitors: Real world evidence from clinical practice in India. J Assoc Physicians India. 2016;64(9):40-4. https://pubmed.ncbi.nlm.nih.gov/27762514.
Ghosh A, Gupta R, Singh P, Dutta A, Misra A. Sodium-glucose cotransporter-2 inhibitors in
patients with type 2 diabetes in North India: A 12-month prospective study in real-world setting. Int J Clin Pract. 2018;72:e13237. https://doi.org/10.1111/ijcp.13237
Wan Seman WJ, Kori N, Rajoo S, et al. Switching from sulphonylurea to a sodium-glucose cotransporter2 inhibitor in the fasting month of Ramadan is associated with a reduction in hypoglycaemia. Diabetes Obes Metab. 2016;18(6):628-32. https://pubmed.ncbi.nlm.nih.gov/26889911. https://doi.org/10.1111/dom.12649.
Aggarwal A, Wadhwa R, Kapoor D, Khanna R. High prevalence of genital mycotic infections with sodium-glucose co-transporter 2 inhibitors among indian patients with type 2 diabetes. Indian J Endocrinol Metab. 2019;23(1):9-13. https://pubmed.ncbi.nlm.nih.gov/31016146. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6446664.
Kohler S, Salsali A, Hantel S, et al. Safety and tolerability of empagliflozin in patients with type 2 diabetes. Clin Ther. 2016;38(6):1299-313. https://pubmed.ncbi.nlm.nih.gov/27085585. https://doi.org/10.1016/j.clinthera.2016.03.031.
Published
How to Cite
Issue
Section
License
Copyright (c) 2022 Ferwani Pankaj M., Maldar Aasim N., Shah N. F., Chauhan Phulrenu H., Chadha Manoj

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
Journal of the ASEAN Federation of Endocrine Societies is licensed under a Creative Commons Attribution-NonCommercial 4.0 International. (full license at this link: http://creativecommons.org/licenses/by-nc/3.0/legalcode).
To obtain permission to translate/reproduce or download articles or use images FOR COMMERCIAL REUSE/BUSINESS PURPOSES from the Journal of the ASEAN Federation of Endocrine Societies, kindly fill in the Permission Request for Use of Copyrighted Material and return as PDF file to jafes@asia.com or jafes.editor@gmail.com.
A written agreement shall be emailed to the requester should permission be granted.









