Efficacy and Safety of Semaglutide for Weight Loss in Obesity Without Diabetes
A Systematic Review and Meta-Analysis
DOI:
https://doi.org/10.15605/jafes.037.02.14Keywords:
obesity, Glucagon-like Peptide -1, weight loss, semaglutideAbstract
Background. The weight loss benefit of semaglutide in patients with diabetes is well-documented, but its clinical utility in treating obesity among patients without diabetes is less described. We therefore assessed the efficacy and safety of subcutaneous semaglutide as treatment for obesity in patients without diabetes.
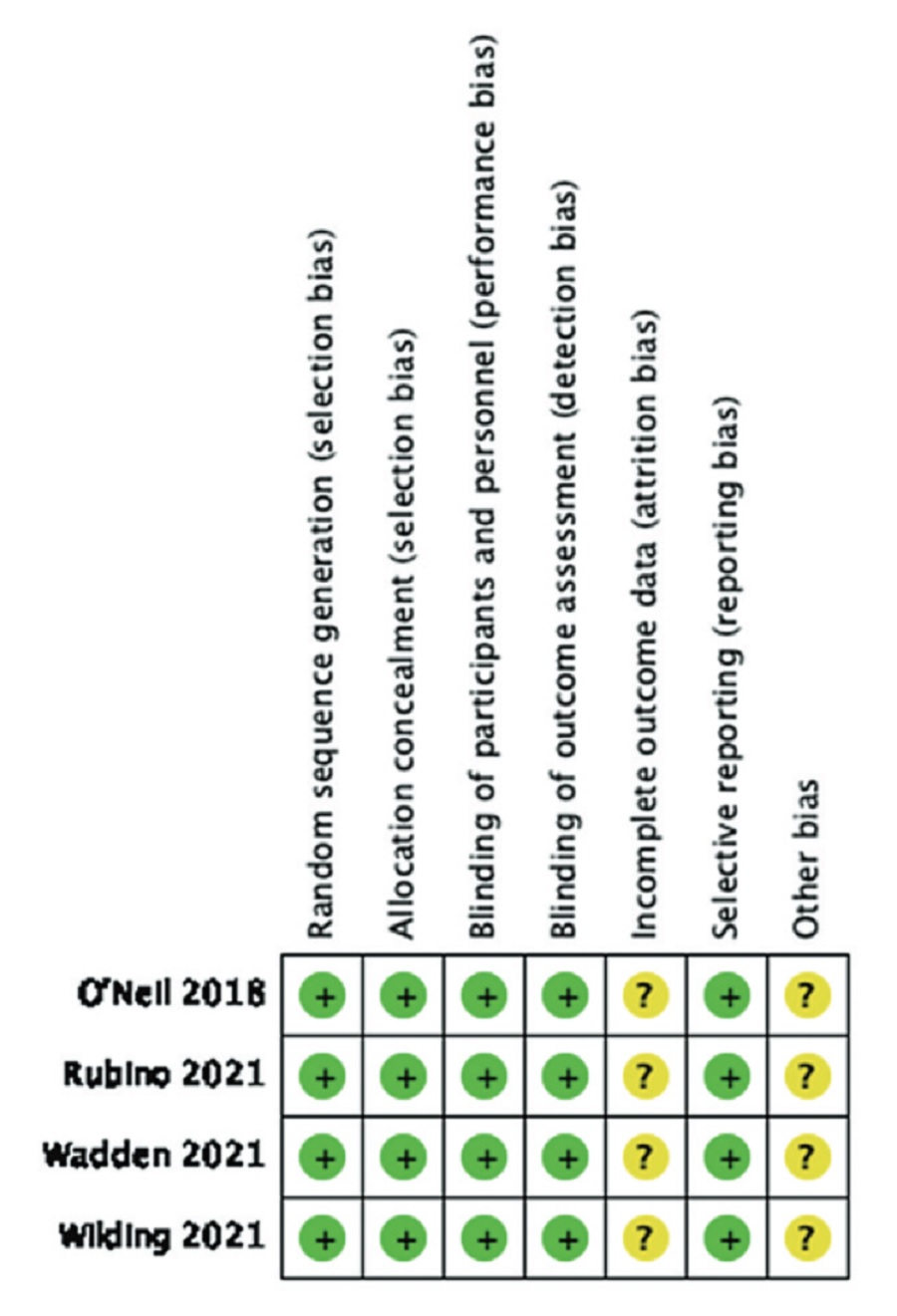
Methodology. A comprehensive search of PubMed/MEDLINE, Cochrane and Google scholar was performed to identify trials on the efficacy and safety of subcutaneous semaglutide on patients with obesity without diabetes. Primary outcome was expressed as percent mean weight difference. Secondary outcomes including risk for gastrointestinal adverse events, discontinuation of treatment and serious adverse events were expressed as risk ratios. These were calculated using the random effects model.
Results. The study included 4 randomized controlled trials having a total of 3,613 individuals with obesity without diabetes. The mean difference for weight reduction was -11.85%, favoring semaglutide [95% confidence interval (CI) (-12.81,-10.90), p<0.00001]. Secondary outcomes showed that the risk of developing gastrointestinal adverse events was 1.59 times more likely with semaglutide (RR 1.59, 95%CI [1.34, 1.88], p<0.00001). Risk for discontinuation due to adverse events was twice as likely in the semaglutide group (RR 2.19, 95%CI [1.36,3.55], p=0.001) and the risk for serious adverse events was 1.6 times more likely for semaglutide (RR1.60, 95% CI [1.24, 2.07], p=0.0003). Serious events were mostly of gastrointestinal and hepatobiliary disorders such as acute pancreatitis and cholelithiasis.
Conclusion. Among individuals with obesity without type 2 diabetes, subcutaneous semaglutide is effective for weight loss with an 11.85% reduction from baseline compared to placebo. This supports the use of semaglutide for weight management in obesity. However, risk of gastrointestinal adverse events, discontinuation of treatment and serious adverse events were higher in the semaglutide group versus placebo.
Downloads
References
Frühbeck G, Busetto L, Dicker D, et al. The ABCD of obesity: An EASO position statement on a diagnostic term with clinical and scientific implications. Obes Facts. 2019;12(2):131–6. https://pubmed.ncbi.nlm.nih.gov/30844811. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6547280. https://doi.org/10.1159/000497124.
World Health Organization. Obesity and overweight. Available from: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight. Accessed 15 May 2021.
Guh DP, Zhang W, Bansback N, Amarsi Z, Birmingham CL, Anis AH. The incidence of co-morbidities related to obesity and overweight: A systematic review and meta-analysis. BMC Public Health. 2009;9:88. https://pubmed.ncbi.nlm.nih.gov/19320986. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2667420. https://doi.org/10.1186/1471-2458-9-88.
van Bloemendaal L, ten Kulve JS, la Fleur SE, Ijzerman RG, Diamant M. Effects of glucagon-like peptide 1 on appetite and body weight: Focus on the CNS. J Endocrinol. 2014;221(1):T1-16. https://pubmed.ncbi.nlm.nih.gov/24323912. https://doi.org/10.1530/JOE-13-0414.
Gloy VL, Briel M, Bhatt DL, et al. Bariatric surgery versus non-surgical treatment for obesity: A systematic review and meta-analysis of randomised controlled trials. BMJ. 2013;347:f5934. https://pubmed.ncbi.nlm.nih.gov/24149519. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3806364. https://doi.org/10.1136/bmj.f5934.
Christou GA, Katsiki N, Blundell J, Fruhbeck G, Kiortsis DN. Semaglutide as a promising antiobesity drug. Obes Rev. 2019;20(6):805–15. https://pubmed.ncbi.nlm.nih.gov/30768766. https://doi.org/10.1111/obr.12839.
Ammori BJ, Skarulis MC, Soran H, Syed AA, Eledrisi M, Malik RA. Medical and surgical management of obesity and diabetes: What’s new? Diabet Med. 2020;37(2):203-10. https://pubmed.ncbi.nlm.nih.gov/31850536. https://doi.org/10.1111/dme.14215.
Dungan KM, Povedano ST, Forst T, et al. Once-weekly dulaglutide versus once-daily liraglutide in metformin-treated patients with type 2 diabetes (AWARD-6): A randomised, open-label, phase 3, non-inferiority trial. Lancet. 2014;384(9951):1349–57. Phttps://pubmed.ncbi.nlm.nih.gov/25018121. https://doi.org/10.1016/S0140-6736(14)60976-4.
Qiao Q, Ouwens MJNM, Grandy S, Johnsson K, Kostev K. Adherence to GLP-1 receptor agonist therapy administered by once-daily or once-weekly injection in patients with type 2 diabetes in Germany. Diabetes Metab Syndr Obes. 2016;9:201-5. https://pubmed.ncbi.nlm.nih.gov/27418849. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4934555. https://doi.org/10.2147/DMSO.S99732.
Johnston SS, Nguyen H, Felber E, et al. Retrospective study of adherence to glucagon-like peptide-1 receptor agonist therapy in patients with type 2 diabetes mellitus in the United States. Adv Ther. 2014;31(11):1119–33. https://pubmed.ncbi.nlm.nih.gov/25408484. https://doi.org/10.1007/s12325-014-0166-0.
Madsbad S. Review of head-to-head comparisons of glucagon-like peptide-1 receptor agonists. Diabetes Obes Metab. 2016;18(4):317-32. https://pubmed.ncbi.nlm.nih.gov/26511102. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5064617. https://doi.org/10.1111/dom.12596.
Dhruv U, Gupta OP. Glucagon like peptide 1 receptor agonists: Glycaemic control and beyond. J Clin Diabetol. 2016;2(4):18–25. http://jcdonline.in/wp-content/uploads/2016/06/6.-JCD_Vol_2_No_4_Urman-Dhruv.pdf.
Capehorn MS, Catarig AM, Furberg JK, et al. Efficacy and safety of once-weekly semaglutide 1.0 mg vs once-daily liraglutide 1.2 mg as add-on to 1–3 oral antidiabetic drugs in subjects with type 2 diabetes (SUSTAIN 10). Diabetes Metab. 2020;46(2):100–9. https://pubmed.ncbi.nlm.nih.gov/31539622. https://doi.org/10.1016/j.diabet.2019.101117.
Finkelstein EA, Trogdon JG, Cohen JW, Dietz W. Annual medical spending attributable to obesity: Payer-and service-specific estimates. 2009;28(5):w822-31. https://pubmed.ncbi.nlm.nih.gov/19635784. https://doi.org/10.1377/hlthaff.28.5.w822.
Magkos F, Fraterrigo G, Okunade AL, et al. Clinical and Translational Report Effects of moderate and subsequent progressive weight loss on metabolic function and adipose tissue biology in humans with obesity. Cell Metab. 2016;23(24):591–601. https://pubmed.ncbi.nlm.nih.gov/26916363. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4833627. https://doi.org/10.1016/j.cmet.2016.02.005.
Jensen MD, Ryan DH, Apovian CM, et al. 2013 AHA/ACC/TOS Guideline for the management of overweight and obesity in adults: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and The Obesity Society. J Am Coll Cardiol. 2014;63(25 Pt 5):2985–3023. https://pubmed.ncbi.nlm.nih.gov/24239920. https://doi.org/10.1016/j.jacc.2013.11.004.
Rubino D, Abrahamsson N, Davies M, et al. Effect of continued weekly subcutaneous semaglutide vs placebo on weight loss maintenance in adults with overweight or obesity: The STEP 4 randomized clinical trial. JAMA. 2021;325(14):1414-25. https://pubmed.ncbi.nlm.nih.gov/33755728. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7988425. https://doi.org/10.1001/jama.2021.3224.
Wilding JPH, Batterham RL, Calanna S, et al. Once-weekly semaglutide in adults with overweight or obesity. N Engl J Med. 2021;384(11):989-1002. https://pubmed.ncbi.nlm.nih.gov/33567185. https://doi.org/10.1056/nejmoa2032183.
Wadden TA, Bailey TS, Billings LK, et al. Effect of subcutaneous semaglutide vs placebo as an adjunct to intensive behavioral therapy on body weight in adults with overweight or obesity: The STEP 3 randomized clinical trial. JAMA - J Am Med Assoc. 2021;325(14):1403-13. https://pubmed.ncbi.nlm.nih.gov/33625476. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7905697. https:10.1001/jama.2021.1831.
O’Neil PM, Birkenfeld AL, McGowan B, et al. Efficacy and safety of semaglutide compared with liraglutide and placebo for weight loss in patients with obesity: A randomised, double-blind, placebo and active controlled, dose-ranging, phase 2 trial. Lancet. 2018;392(10148):637-49. https://doi.org/10.1016/S0140-6736(18)31773-2.
Published
How to Cite
Issue
Section
License
Copyright (c) 2022 Hanna Clementine Tan, Oliver Allan Dampil, Maricar Mae Marquez

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
The full license is at this link: http://creativecommons.org/licenses/by-nc/3.0/legalcode).
To obtain permission to translate/reproduce or download articles or use images FOR COMMERCIAL REUSE/BUSINESS PURPOSES from the Journal of the ASEAN Federation of Endocrine Societies, kindly fill in the Permission Request for Use of Copyrighted Material and return as PDF file to jafes@asia.com or jafes.editor@gmail.com.
A written agreement shall be emailed to the requester should permission be granted.










