Real-World Use of Once-Weekly Semaglutide in Thai Patients with Type 2 Diabetes Mellitus in a Private Hospital Setting
DOI:
https://doi.org/10.15605/jafes.038.01.11Keywords:
semaglutide, once-weekly, real-world, ThaiAbstract
Objective. To evaluate the real-world use of once-weekly semaglutide among Thai patients with type 2 diabetes (T2DM) in a private hospital setting.
Methodology. A retrospective review of Thai patients with T2DM who have initiated semaglutide for at least 1 month between June 2020 and March 2022 at Theptarin Hospital, Bangkok, Thailand.
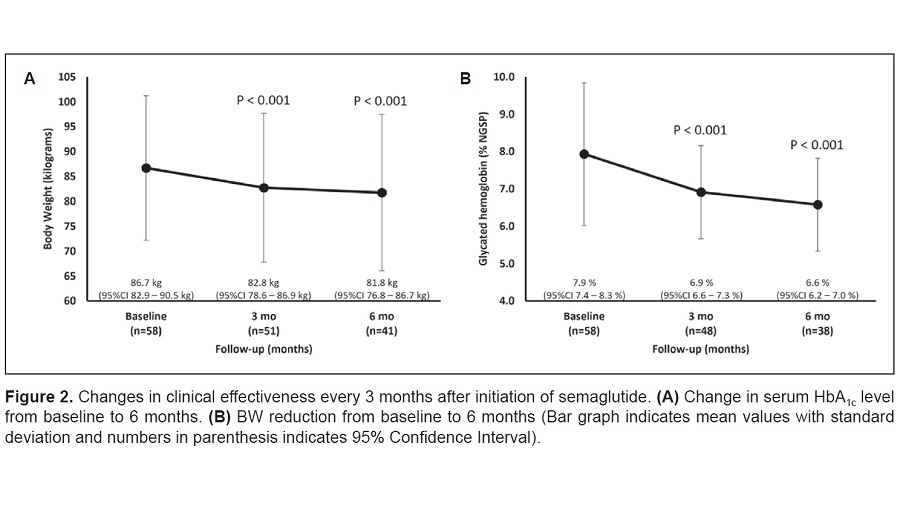
Results. A total of 58 patients (50% female, mean age 55.6 ± 15.9 years, with duration of diabetes 12.6 ± 10.3 years, BMI 31.5 ± 4.4 kg/m2, baseline HbA1c 7.9 ± 1.9%, with prior GLP-1 RA use 24.1%, and concomitant SGLT2i intake (41.4%) were included. During a median follow-up of 6 months, the mean serum HbA1c level reduction was 1.3 ± 1.7% with weight loss of 4.7 ± 4.1 kg. The proportion of patients who achieved optimal and sustainable glycemic control (HbA1c <7.0%) increased from 43.1% to 55.8% at the last follow-up. The proportion of patients reaching both HbA1c targets of <7.0% and 5% weight loss was 27.8%. No cases of pancreatitis, cancer, or progressive retinopathy were observed.
Conclusions. In this single center undertaking, it was shown that in among persons with T2DM and obesity in Thailand, semaglutide was associated with short-term glycemic control and weight loss comparable with what has been observed in randomized clinical trials and other RWE.
Downloads
References
American Diabetes Association; Professional Practice Committee. 9. Pharmacologic approaches to glycemic treatment: Standards of Medical Care in Diabetes—2022. Diabetes Care. 2022;45(Suppl 1):S125-43. https://pubmed.ncbi.nlm.nih.gov/34964831. https://doi.org/10.2337/dc22-s009.
Davies MJ, Aroda VR, Collins BS, et al. Management of hyperglycemia in type 2 diabetes, 2022. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care. 2022;45(11):2753-86. https://pubmed.ncbi.nlm.nih.gov/36148880. https://doi.org/10.2337/dci22-0034.
Chan JC, Malik V, Jia W, et al. Diabetes in Asia: epidemiology, risk factors, and pathophysiology. JAMA. 2009;301(20):2129-40. https://pubmed.ncbi.nlm.nih.gov/19470990. https://doi.org/10.1001/jama.2009.726
Møller JB, Pedersen M, Tanaka H, et al. Body composition is the main determinant for the difference in type 2 diabetes pathophysiology between Japanese and Caucasians. Diabetes Care. 2014;37(3):796-804. Phttps://pubmed.ncbi.nlm.nih.gov/24130359. https://doi.org/10.2337/dc13-0598.
Sherman RE, Anderson SA, Dal Pan GJ, et al. Real-world evidence - what is it and what can it tell us? N Engl J Med. 2016;375(23):2293-7. https://pubmed.ncbi.nlm.nih.gov/27959688. https://doi.org/10.1056/nejmsb1609216.
Aroda VR, Ahmann A, Cariou B, et al. Comparative efficacy, safety, and cardiovascular outcomes with once-weekly subcutaneous semaglutide in the treatment of type 2 diabetes: Insights from the SUSTAIN 1-7 trials. Diabetes Metab. 2019;45(5):409-18. https://pubmed.ncbi.nlm.nih.gov/30615985. https://doi.org/10.1016/j.diabet.2018.12.001.
Marso SP, Bain SC, Consoli A, et al. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2016;375(19):1834-44. https://pubmed.ncbi.nlm.nih.gov/27633186. https://doi.org/10.1056/nejmoa1607141
Rudofsky G, Catarig AM, Favre L, et al. Real-world use of once-weekly semaglutide in patients with type 2 diabetes: Results from the SURE Switzerland multicentre, prospective, observational study. Diabetes Res Clin Pract. 2021;178: 108931. https://pubmed.ncbi.nlm.nih.gov/34217773. https://doi.org/10.1016/j.diabres.2021.108931
Rajamand Ekberg N, Bodholdt U, Catarig AM, et al. Real-world use of once-weekly semaglutide in patients with type 2 diabetes: Results from the SURE Denmark/Sweden multicentre, prospective, observational study. Prim Care Diabetes. 2021;15(5):871-8. https://pubmed.ncbi.nlm.nih.gov/34183269. https://doi.org/10.1016/j.pcd.2021.06.008.
Holmes P, Bell HE, Bozkurt K, et al. Real-world use of once-weekly semaglutide in type 2 diabetes: Results from the SURE UK multicentre, prospective, observational study. Diabetes Ther. 2021;12(11):2891-905. https://pubmed.ncbi.nlm.nih.gov/34562237. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8475854. https://doi.org/10.1007/s13300-021-01141-8.
Yale JF, Catarig AM, Grau K, et al. Use of once-weekly semaglutide in patients with type 2 diabetes in routine clinical practice: Results from the SURE Canada multicentre, prospective, observational study. Diabetes Obes Metab. 2021;23(10):2269-78. https://pubmed.ncbi.nlm.nih.gov/34142429. https://doi.org/10.1111/dom.14468.
Yale JF, Bodholdt U, Catarig AM, et al. Real-world use of once-weekly semaglutide in patients with type 2 diabetes: Pooled analysis of data from four SURE studies by baseline characteristic subgroups. BMJ Open Diabetes Res Care. 2022;10(2):e002619. https://pubmed.ncbi.nlm.nih.gov/35383100. https://doi.org/10.1136/bmjdrc-2021-002619.
Visaria J, Uzoigwe C, Swift C, Dang-Tan T, Paprocki Y, Willey VJ. Real-world effectiveness of once-weekly semaglutide from a US commercially insured and medicare advantage population. Clin Ther. 2021;43(5):808-21. https://pubmed.ncbi.nlm.nih.gov/33785221. https://doi.org/10.1016/j.clinthera.2021.03.003.
Williams DM, Ruslan AM, Khan R, et al. Real-world clinical experience of semaglutide in secondary care diabetes: A retrospective observational study. Diabetes Ther. 2021;12(3):801-11. https://pubmed.ncbi.nlm.nih.gov/33565043. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7872110. https://doi.org/10.1007/s13300-021-01015-z.
Hansen KB, Svendstrup M, Lund A, Knop FK, Vilsbøll T, Vestergaard H. Once-weekly subcutaneous semaglutide treatment for persons with type 2 diabetes: Real-world data from a diabetes out-patient clinic. Diabet Med. 2021;38(10):e14655. https://pubmed.ncbi.nlm.nih.gov/34291491. https://doi.org/10.1111/dme.14655.
Di Loreto C, Minarelli V, Nasini G, Norgiolini R, Del Sindaco P. Effectiveness in real world of once-weekly semaglutide in people with type 2 diabetes: Glucagon-like peptide receptor agonist naïve or switchers from other glucagon-like peptide receptor agonists: Results from a retrospective observational study in Umbria. Diabetes Ther. 2022;13(3):551-67. https://pubmed.ncbi.nlm.nih.gov/35230650. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8886341. https://doi.org/10.1007/s13300-022-01218-y.
Marzullo P, Daffara T, Mele C, et al. Real-world evaluation of weekly subcutaneous treatment with semaglutide in a cohort of Italian diabetic patients. J Endocrinol Invest. 2022;45(8):1587-98. https://pubmed.ncbi.nlm.nih.gov/35429298. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9270295. https://doi.org/10.1007/s40618-022-01799-2.
Garcia de Lucas MD, Miramontes-González JP, Avilés-Bueno B, Jiménez-Millán AI, Rivas-Ruiz F, Pérez-Belmonte LM. Real-world use of once-weekly semaglutide in patients with type 2 diabetes at an outpatient clinic in Spain. Front Endocrinol (Lausanne). 2022;13: 995646. https://pubmed.ncbi.nlm.nih.gov/36187123. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9523693. https://doi.org/10.3389/fendo.2022.995646
Gabery S, Salinas CG, Paulsen SJ, et al. Semaglutide lowers body weight in rodents via distributed neural pathways. JCI Insight 2020;5(6): e133429. https://pubmed.ncbi.nlm.nih.gov/32213703. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7213778. https://doi.org/10.1172/jci.insight.133429.
Lingvay I, Hansen T, Macura S, et al. Superior weight loss with once-weekly Semaglutide versus other glucagon-like peptide-1 receptor agonists is independent of gastrointestinal adverse events. BMJ Open Diabetes Res Care. 2020;8(2): e001706. https://pubmed.ncbi.nlm.nih.gov/33115821. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7594204. https://doi.org/10.1136/bmjdrc-2020-001706.
Weeda ER, Muraoka AK, Brock MD, Cannon JM. Medication adherence to injectable glucagon-like peptide-1 (GLP-1) receptor agonists dosed once-weekly vs once daily in patients with type 2 diabetes: A meta-analysis. Int J Clin Pract. 2021;75(9): e14060. https://pubmed.ncbi.nlm.nih.gov/33527605. https://doi.org/10.1111/ijcp.14060.
DeFronzo RA, Inzucchi S, Abdul-Ghani M, Nissen SE. Pioglitazone: The forgotten, cost-effective cardioprotective drug for type 2 diabetes. Diab Vasc Dis Res. 2019; 16(2):133-43. https://pubmed.ncbi.nlm.nih.gov/30706731. https://doi.org/10.1177/1479164118825376.
Jabbour SA, Frías JP, Ahmed A, et al. Efficacy and safety over 2 years of exenatide plus dapagliflozin in the DURATION-8 study: A multicenter, double-blind, phase 3, randomized controlled trial. Diabetes Care. 2020;43(10):2528-36. https://pubmed.ncbi.nlm.nih.gov/32816874. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7510043. https://doi.org/10.2337/dc19-1350.
Goncalves E, Bell DSH. Glucagon-like peptide-1 receptor agonists and sodium-glucose co-transporter-2 inhibitors: Sequential or simultaneous start? Diabetes Obes Metab. 2017;19(6):909-11. https://pubmed.ncbi.nlm.nih.gov/28176440. https://doi.org/10.1111/dom.12897.
Li C, Luo J, Jiang M, Wang K. The efficacy and safety of the combination therapy with GLP-1 receptor agonists and SGLT-2 inhibitors in type 2 diabetes mellitus: A systematic review and meta-analysis. Front Pharmacol. 2022;13: 838277. https://pubmed.ncbi.nlm.nih.gov/35185588. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8854770. https://doi.org/10.3389/fphar.2022.838277.
Brown RE, Bech PG, Aronson R. Semaglutide once weekly in people with type 2 diabetes: Real-world analysis of the Canadian LMC diabetes registry (SPARE study). Diabetes Obes Metab. 2020;22(11):2013-20. https://pubmed.ncbi.nlm.nih.gov/32538541. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7689820. https://doi.org/10.1111/dom.14117.
Global Health & Population Project on Access to Care for Cardiometabolic Diseases (HPACC). Expanding access to newer medicines for people with type 2 diabetes in low-income and middle-income countries: A cost-effectiveness and price target analysis. Lancet Diabetes Endocrinol. 2021;9(12):825-36. https://pubmed.ncbi.nlm.nih.gov/34656210. https://doi.org/10.1016/s2213-8587(21)00240-0.
Blonde L, Khunti K, Harris SB, Meizinger C, Skolnik NS. Interpretation and impact of real-world clinical data for the practicing clinician. Adv Ther. 2018;35(11):1763-74. https://pubmed.ncbi.nlm.nih.gov/30357570. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6223979. https://doi.org/10.1007/s12325-018-0805-y.
Published
How to Cite
Issue
Section
License
Copyright (c) 2023 Yotsapon Thewjitcharoen, Nalin Yenseung, Siriwan Butadej, Soontaree Nakasatien, Phawinpon Chotwanvirat, Waralee Chatchomchuan, Ekgaluck Wanothayaroj, Sirinate Krittiyawong, Thep Himathongkam

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
The full license is at this link: http://creativecommons.org/licenses/by-nc/3.0/legalcode).
To obtain permission to translate/reproduce or download articles or use images FOR COMMERCIAL REUSE/BUSINESS PURPOSES from the Journal of the ASEAN Federation of Endocrine Societies, kindly fill in the Permission Request for Use of Copyrighted Material and return as PDF file to jafes@asia.com or jafes.editor@gmail.com.
A written agreement shall be emailed to the requester should permission be granted.










