Profile of Levothyroxine Replacement Therapy in Graves’ Disease Patients with Hypothyroidism Post-Radioactive Iodine Ablation
Focus on Different Weight-Based Regimens
DOI:
https://doi.org/10.15605/jafes.037.01.19Keywords:
post-ablative, hypothyroidism, Graves' disease, in-range TSH, levothyroxine (LT4), lean body massAbstract
Objective. To evaluate the status of euthyroidism achieved among Thai patients with post-ablative hypothyroidism and to examine the difference between various weight-based daily levothyroxine (LT4) replacement regimens in these patients.
Methodology. We conducted a retrospective review of Thai patients with Graves’ disease (GD) who developed hypothyroidism following radioactive iodine treatment from 2016 to 2020 at Theptarin hospital. Daily LT4 dose was calculated based on actual body weight (ABW), ideal body weight (IBW), and estimated lean body mass (LBM).
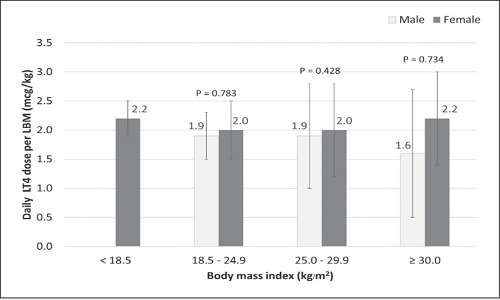
Results. We reviewed a total of 271 patient records. Of these, 81.2% were females with a mean age of 40.8±11.7 years, LT4 intake duration of 27.1±14.6 months, and LT4 dose/kg ABW of 1.4±0.5 μg/kg/day. At the final follow-up, 62.4% of patients achieved thyroid-stimulating hormone (TSH) levels within the reference interval, 15.5% had TSH levels over, and 22.1% had TSH levels under the reference range. Obese patients required a lower daily LT4 dose relative to ABW and higher daily LT4 dose relative to IBW to attain euthyroidism (ABW 1.1±0.4 μg/kg/day and IBW 2.0±0.8 μg/kg/day). Estimated daily LT4 dose based on LBM showed a constant dosage of 2.0 μg/kg/day in all BMI categories.
Conclusions. Suboptimum LT4 replacement therapy was found in almost half of hypothyroid patients with GD treated with radioactive iodine. Estimated LBM was a better indicator for dosing calculation in these patients compared with ABW and IBW.
Downloads
References
Ross DS, Burch HB, Cooper DS, et al. 2016 American Thyroid Association guidelines for diagnosis and Management of Hyperthyroidism and Other Causes of thyrotoxicosis. Thyroid. 2016;26(10): 1343–421. https://pubmed.ncbi.nlm.nih.gov/27521067. https://doi.org/10.1089/thy.2016.0229.
Thewjitcharoen Y, Karndumri K, Chatchomchuan W, et al. Practice patterns and outcomes in the management of Thai patients with Graves' disease. Thyroid Res. 2021;14(1):5. https://pubmed.ncbi.nlm.nih.gov/33658045. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7927256. https://doi.org/10.1186/s13044-021-00097-y.
Biondi B, Cooper DS. Thyroid hormone therapy for hypothyroidism. Endocrine. 2019;66(1):18-26. https://pubmed.ncbi.nlm.nih.gov/31372822. https://doi.org/10.1007/s12020-019-02023-7.
Garber J, Cobin R, Gharib H, et al. Clinical practice guidelines for hypothyroidism in adults: Cosponsored by the American Association of Clinical Endocrinologists and the American Thyroid Association. Thyroid. 2012;22(12):1200-35. https://pubmed.ncbi.nlm.nih.gov/22954017. https://doi.org/10.1089/thy.2012.0205.
Jonklaas J. Optimal thyroid hormone replacement. Endocr Rev. 2020;43(2):366-404. https://pubmed.ncbi.nlm.nih.gov/34543420. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8905334 (available on 2022-09-20). https://doi.org/10.1210/endrev/bnab031.
Gordon MB, Gordon MS. Variations in adequate levothyroxine replacement therapy in patients with different causes of hypothyroidism. Endocr Pract. 1999;5(5):233-8. https://pubmed.ncbi.nlm.nih.gov/15251659. https://doi.org/10.4158/ep.5.5.233.
Roos A, Linn-Rasker SP, van Domburg RT, Tijssen JP, Berghout A. The starting dose of levothyroxine in primary hypothyroidism treatment: A prospective, randomized, double-blind trial. Arch Intern Med. 2005;165(15):1714-20. https://pubmed.ncbi.nlm.nih.gov/16087818. https://doi.org/10.1001/archinte.165.15.1714.
Rajatanavin R, Chailurkit L, Kongsuksai A, Chalayondeja W, Sirisriro R, Choksuwatanasakul P. Optimum replacement, suppressive doses and bioavailability of levothyroxine therapy in Thai patients. Intern Med. 1990;6:128-34.
Tan NC, Chew RQ, Koh YLE, et al. Primary hypothyroidism in the community: Lower daily dosages of levothyroxine replacement therapy for Asian patients. Medicine (Baltimore). 2017;96(7):e6145. https://pubmed.ncbi.nlm.nih.gov/28207545. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5319534. https://doi.org/10.1097/md.0000000000006145.
Ratanapornsompong G, Sriphrapradang C. Appropriate dose of levothyroxine replacement therapy for hypothyroid obese patients. J Clin Transl Endocrinol. 2021;25:100264. https://pubmed.ncbi.nlm.nih.gov/34401353. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8350177. https://doi.org/10.1016/j.jcte.2021.100264.
Vaidya B, Pearce SH. Management of hypothyroidism in adults. BMJ. 2008;337:a801. https://pubmed.ncbi.nlm.nih.gov/18662921. https://doi.org/10.1136/bmj.a801.
Okosieme O, Gilbert J, Abraham P, et al. Management of primary hypothyroidism: Statement by the British Thyroid Association Executive Committee. Clin Endocrinol (Oxf). 2016;84(6):799-808. https://pubmed.ncbi.nlm.nih.gov/26010808. https://doi.org/10.1111/cen.12824.
Somwaru LL, Arnold AM, Joshi N, Fried LP, Cappola AR. High frequency of and factors associated with thyroid hormone over-replacement and under-replacement in men and women aged 65 and over. J Clin Endocrinol Metab. 2009;94(4):1342-5. https://pubmed.ncbi.nlm.nih.gov/19126628. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2682480. https://doi.org/10.1210/jc.2008-1696.
Flynn RW, Bonellie SR, Jung RT, MacDonald TM, Morris AD, Leese GP. Serum thyroid-stimulating hormone concentration and morbidity from cardiovascular disease and fractures in patients on long-term thyroxine therapy. J Clin Endocrinol Metab. 2010;95(1):186-93. https://pubmed.ncbi.nlm.nih.gov/19906785. https://doi.org/10.1210/jc.2009-1625.
Thayakaran R, Adderley NJ, Sainsbury C, et al. Thyroid replacement therapy, thyroid stimulating hormone concentrations, and long-term health outcomes in patients with hypothyroidism: Longitudinal study. BMJ. 2019;366:l4892. https://pubmed.ncbi.nlm.nih.gov/31481394. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6719286. https://doi.org/10.1136/bmj.l4892.
Scargill JJ, Livingston M, Holland D, Duff CJ, Fryer AA, Heald AH. Monitoring thyroid function in patients on levothyroxine. Assessment of conformity to national guidance and variability in practice. Exp Clin Endocrinol Diabetes. 2017;125(9):625-33. https://pubmed.ncbi.nlm.nih.gov/28407667. https://doi.org/10.1055/s-0043-103018.
Yavuz DG, Yazıcı D, Keskin L, et al. Out-of-reference range thyroid-stimulating hormone levels in levothyroxine-treated primary hypothyroid patients: A multicenter observational study. Front Endocrinol (Lausanne). 2017;8:215. https://pubmed.ncbi.nlm.nih.gov/28955301. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5600911. https://doi.org/10.3389/fendo.2017.00215.
Shim J, Lin T, Dashiell-Earp C, Nechrebecki M, Leung AM. Endocrinology practice patterns of hypothyroidism and osteoporosis management in a U.S. tertiary academic medical center. Clin Diabetes Endocrinol. 2019;5:10. https://pubmed.ncbi.nlm.nih.gov/31360539. https://doi.org/10.1186/s40842-019-0085-8.
Cunningham JJ, Barzel US. Lean body mass is a predictor of the daily requirement for thyroid hormone in older men and women. J Am Geriatr Soc. 1984;32(3):204-7. https://pubmed.ncbi.nlm.nih.gov/6699335. https://doi.org/10.1111/j.1532-5415.1984.tb02003.x.
Santini F, Pinchera A, Marsili A, et al. Lean body mass is a major determinant of levothyroxine dosage in the treatment of thyroid diseases. J Clin Endocrinol Metab. 2005;90(1):124-7. https://pubmed.ncbi.nlm.nih.gov/15483074. https://doi.org/10.1210/jc.2004-1306
Morgan DJ, Bray KM. Lean body mass as a predictor of drug dosage. Implications for drug therapy. Clin Pharmacokinet. 1994;26(4):292-307. https://pubmed.ncbi.nlm.nih.gov/8013162. https://doi.org/10.2165/00003088-199426040-00005.
Papoian V, Ylli D, Felger EA, Wartofsky L, Rosen JE. Evaluation of thyroid hormone replacement dosing in overweight and obese patients after a thyroidectomy. Thyroid. 2019;29(11):1558-1562. https://pubmed.ncbi.nlm.nih.gov/31573413. https://doi.org/10.1089/thy.2019.0251.
Jin J, Allemang MT, McHenry CR. Levothyroxine replacement dosage determination after thyroidectomy. Am J Surg. 2013;205(3):360-3. https://pubmed.ncbi.nlm.nih.gov/23369308. https://doi.org/10.1016/j.amjsurg.2012.10.015.
Hume R. Prediction of lean body mass from height and weight. J Clin Pathol. 1966;19(4):389-91. https://pubmed.ncbi.nlm.nih.gov/5929341. https://doi.org/10.1136/jcp.19.4.389.
Pai MP, Paloucek FP. The origin of the "ideal" body weight equations. Ann Pharmacother. 2000;34(9):1066-9. https://pubmed.ncbi.nlm.nih.gov/10981254. https://doi.org/10.1345/aph.19381.
Centanni M, Gargano L, Canettieri G, et al. Thyroxine in goiter, Helicobacter pylori infection, and chronic gastritis. N Engl J Med. 2006;354(17):1787-95. https://pubmed.ncbi.nlm.nih.gov/16641395. https://doi.org/10.1056/nejmoa043903.
Devdhar M, Drooger R, Pehlivanova M, Singh G, Jonklaas J. Levothyroxine replacement doses are affected by gender and weight, but not age. Thyroid. 2011;21(8):821-7. https://pubmed.ncbi.nlm.nih.gov/21751885. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3148125. https://doi.org/10.1089/thy.2011.0029.
Caron P, Grunenwald S, Persani L, Borson-Chazot F, Leroy R, Duntas L. Factors influencing the levothyroxine dose in the hormone replacement therapy of primary hypothyroidism in adults. Rev Endocr Metab Disord. 2021:1-21. https://pubmed.ncbi.nlm.nih.gov/34671932. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8528480. https://doi.org/10.1007/s11154-021-09691-9.
Effraimidis G, Watt T, Feldt-Rasmussen U. Levothyroxine therapy in elderly patients with hypothyroidism. Front Endocrinol (Lausanne). 2021;12:641560. https://pubmed.ncbi.nlm.nih.gov/33790867. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8006441. https://doi.org/10.3389/fendo.2021.641560.
Benvenga S, Carlé A. Levothyroxine formulations: Pharmacological and clinical implications of generic substitution. Adv Ther. 2019;36(Suppl 2):59-71. https://pubmed.ncbi.nlm.nih.gov/31485974. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6822816. https://doi.org/10.1007/s12325-019-01079-1.
Published
How to Cite
Issue
Section
License
The full license is at this link: http://creativecommons.org/licenses/by-nc/3.0/legalcode).
To obtain permission to translate/reproduce or download articles or use images FOR COMMERCIAL REUSE/BUSINESS PURPOSES from the Journal of the ASEAN Federation of Endocrine Societies, kindly fill in the Permission Request for Use of Copyrighted Material and return as PDF file to jafes@asia.com or jafes.editor@gmail.com.
A written agreement shall be emailed to the requester should permission be granted.










