Neonatal Outcomes of Pregnancies Complicated by Maternal Hyperthyroidism
DOI:
https://doi.org/10.15605/jafes.037.02.03Keywords:
Infant of mother with maternal hyperthyroidism, maternal hyperthyroidism, Graves’ disease(GD), Thyroid function test(TFT)Abstract
Objective. This study aimed to determine the proportion, clinical characteristics, hormonal status, median time for normalization of serum thyroxine (FT4) and thyroid-stimulating hormone (TSH) and factors affecting time to thyroid function test (TFT) normalization of neonates born to mothers with maternal hyperthyroidism admitted in our institution.
Methodology. This was a retrospective cohort study that included 170 newborns admitted to the Neonatal Intensive Care Unit (NICU) of Hospital Universiti Sains Malaysia (HUSM) with a history of maternal hyperthyroidism from January 2013 until December 2018. We analyzed their baseline demographic and clinical characteristics, maternal thyroid status and antibody levels. Finally, we analyzed newborn thyroid function and thyroid antibodies.
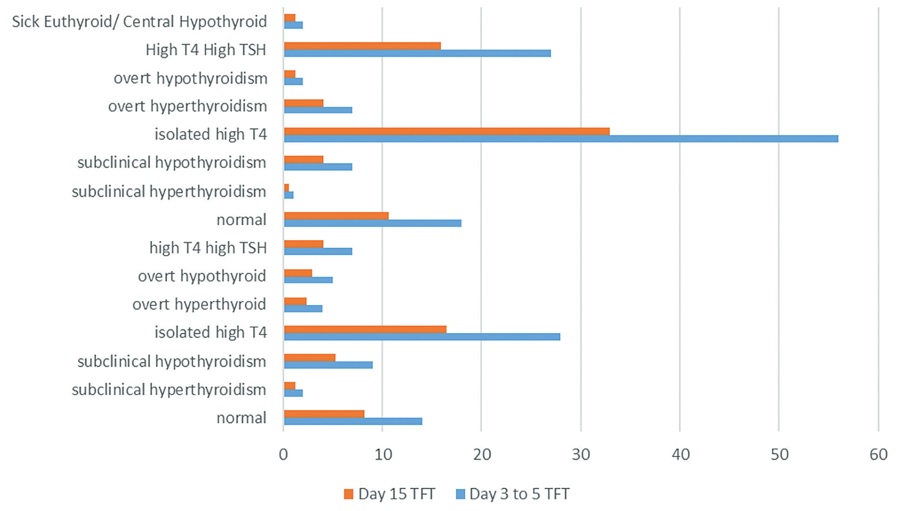
Results. The proportion of neonates born to mothers with maternal hyperthyroidism was 0.8% (170 of 20198 neonates within the study period). Seven (4.1%) developed overt hyperthyroidism, while four (2.4%) had thyroid storm. The median time for thyroid function test normalization was 30 days (95% CI: 27.1 to 32.8). The median time for TFT normalization was longer among neonates of mothers with positive thyroid antibodies [46.6 days ( 39.4)] and of mothers who received anti-thyroid treatment [31.7 days (95% CI, 23.5 to 39.9)].
Conclusion. Neonates born to mothers with hyperthyroidism is uncommon. These babies were observed to have a longer time for normalization of thyroid function tests if their mothers had thyroid antibodies or received anti-thyroid treatment.
Downloads
References
Polak M, Le Gac I, Vuillard E, et al. Fetal and neonatal thyroid function in relation to maternal Graves’ disease. Best Pract Res Clin Endocrinol Metab. 2004;18(2):289-302. https://pubmed.ncbi.nlm.nih.gov/15157841. https://doi.org/10.1016/j.beem.2004.03.009.
Wong SLJ, Jalaludin MY, Zaini AA, Samingan N, Harun F. Congenital hypothyroidism: An audit and study of different cord blood screening TSH values in a tertiary medical centre in Malaysia. Advances in Endocrinology 2015;2015:387684. https://doi.org/10.1155/2015/387684.
Léger J. Management of fetal and neonatal Graves’ disease. Horm Res Paediatr. 2017;87(1):1-6. https://pubmed.ncbi.nlm.nih.gov/27978517. https://doi.org/10.1159/000453065.
Laurberg P, Andersen SL. Endocrinology in pregnancy: Pregnancy and the incidence, diagnosing and therapy of Graves’ disease. Eur J Endocrinol. 2016;175(5):R219-30. https://pubmed.ncbi.nlm.nih.gov/27280373. https://doi.org/10.1530/EJE-16-0410.
Dulek H, Vural F, Aka N, Zengin S. The prevalence of thyroid dysfunction and relationship with perinatal outcomes in the third trimester. North Clin Istanb. 2019;6(3):267-72. https://pubmed.ncbi.nlm.nih.gov/31650114. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6790929. https://doi.org/10.14744/nci.2018.51422.
Lim BH, Raman S, Sivanesaratnam V, Ngan A. Thyrotoxicosis in pregnancy—A six year review. Singapor Med J. 1989;30(6):539-41. https://pubmed.ncbi.nlm.nih.gov/2635396.
Deng F, Yang ZY, Zhang YP, Wang YL, Hu JY, Zhang F. TSH adenoma and syndrome of resistance to thyroid hormones—Two cases report of syndrome of inappropriate secretion of thyrotropin. Brain Behav. 2021;11(5):e02081. https://pubmed.ncbi.nlm.nih.gov/33751836. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8119795. https://doi.org/10.1002/brb3.2081.
Li C, Zhou J, Huang Z, et al. The clinical value and variation of antithyroid antibodies during pregnancy. Dis Markers. 2020;2020:8871951. https://pubmed.ncbi.nlm.nih.gov/33144894. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7599418. https://doi.org/10.1155/2020/8871951.
Chapman AK, Farmer ZJ, Mastrandrea LD, Matlock KA. Neonatal thyroid function and disorders. Clin Obstet Gynecol. 2019;62(2):373-87. https://pubmed.ncbi.nlm.nih.gov/31026231. https://doi.org/10.1097/GRF.0000000000000434.
Papendieck P, Chiesa A, Prieto L, Gruñeiro-Papendieck L. Thyroid disorders of neonates born to mothers with Graves’ disease. 2009;22(6):547-53. https://pubmed.ncbi.nlm.nih.gov/19694202. https://doi.org/10.1515/jpem.2009.22.6.547.
Kahaly GJ, Bartalena L, Hegedüs L, Leenhardt L, Poppe K, Pearce SH. 2018 European Thyroid Association Guideline for the management of Graves’ hyperthyroidism. Eur Thyroid J. 2018;7(4):167-86. https://pubmed.ncbi.nlm.nih.gov/30283735. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6140607. https://doi.org/10.1159/000490384.
Carmen MCT, Martín MJR, Ruiz JJ, Segura SA. Maternal autoimmune thyroid disease: Relevance for the newborn. Med Clin (Barc). 2015;144(7):297-303. https://pubmed.ncbi.nlm.nih.gov/24486115. https://doi.org/10.1016/j.medcli.2013.10.024.
Levy-Shraga Y, Tamir-Hostovsky L, Boyko V, Lerner-Geva L, Pinhas-Hamiel O. Follow-up of newborns of mothers with Graves’ disease. Thyroid. 2014;24(6):1032-9. https://pubmed.ncbi.nlm.nih.gov/24472020. https://doi.org/10.1089/thy.2013.0489.
Männistö T, Mendola P, Reddy U, Laughon SK. Neonatal outcomes and birth weight in pregnancies complicated by maternal thyroid disease. Am J Epidemiol. 2013;178(5):731-40. https://pubmed.ncbi.nlm.nih.gov/23666815. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3755642. https://doi.org/10.1093/aje/kwt031.
van der Kaay DCM, Wasserman JD, Palmert MR. Management of neonates born to mothers with Graves’ disease. 2016;137(4):e20151878. https://pubmed.ncbi.nlm.nih.gov/26980880. https://doi.org/10.1542/peds.2015-1878.
Miller A, Silver KD. Thyroid storm with multiorgan failure treated with plasmapheresis. Case Rep Endocrinol. 2019;2019:2475843. https://pubmed.ncbi.nlm.nih.gov/31687222. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6811794. https://doi.org/10.1155/2019/2475843.
Samuels SL, Namoc SM, Bauer AJ. Neonatal thyrotoxicosis. Clin Perinatol. 2018;45(1):31-40. https://pubmed.ncbi.nlm.nih.gov/29406005. https://doi.org/10.1016/j.clp.2017.10.001.
Lee ML, Wang YM, Chang MC. Concurrence of persistent pulmonary hypertension of the newborn, myocardial ischemia, supraventricular tachycardia, and congestive heart failure as a harbinger of neonatal Graves’ disease. Acta Cardiol Sin. 2020;36(3):272-5. https://pubmed.ncbi.nlm.nih.gov/32425443. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7220969. https://doi.org/10.6515/ACS.202005_36(3).20200105A
Banigé M, Polak M, Luton D, Research Group for Perinatal Dysthyroidism (RGPD) Study Group. Prediction of neonatal hyperthyroidism. J Pediatr. 2018;197:249-254.e1. https://pubmed.ncbi.nlm.nih.gov/29605392. https://doi.org/10.1016/j.jpeds.2018.01.071.
Özon A, Tekin N, Şıklar Z, et al. Neonatal effects of thyroid diseases in pregnancy and approach to the infant with increased TSH: Turkish Neonatal and Pediatric Endocrinology and Diabetes Societies consensus report. Turk Pediatri Ars. 2018;53(Suppl 1):S209-23. https://pubmed.ncbi.nlm.nih.gov/31236034. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6568290. https://doi.org/10.5152/TurkPediatriArs.2018.01819.
Besançon A, Beltrand J, Le Gac I, Luton D, Polak M. Management of neonates born to women with Graves’ disease: A cohort study. Eur J Endocrinol. 2014;170(6):855-62. https://pubmed.ncbi.nlm.nih.gov/24670885. https://doi.org/10.1530/EJE-13-0994.
Polak M, Van Vliet G. Therapeutic approach of fetal thyroid disorders. Hormone Res Paediatr. 2010;74(1):1-5. https://pubmed.ncbi.nlm.nih.gov/20453471. https://doi.org/10.1159/000297595.
Rovelli R, Vigone MC, Giovanettoni C, et al. Newborn of mothers affected by autoimmune thyroiditis: The importance of thyroid function monitoring in the first months of life. Ital J Pediatr. 2010;36:24. https://pubmed.ncbi.nlm.nih.gov/20219125. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2851706. https://doi.org/10.1186/1824-7288-36-24.
Segni M. Neonatal hyperthyroidism. In: Feingold KR, Anawalt B, Boyce A, et al., eds. Endotext [Internet]. South Dartmouth (MA): MDText.com, Inc.; 2019. https://www.ncbi.nlm.nih.gov/books/NBK279019/.
Chen X, Jin B, Xia J, et al. Effects of thyroid peroxidase antibody on maternal and neonatal outcomes in pregnant women in an iodine-sufficient area in China. Int J Endocrinol. 2016;2016:6461380. https://pubmed.ncbi.nlm.nih.gov/26884759. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4738937. https://doi.org/10.1155/2016/6461380.
Wada M, Kita M, Kawasaki K, et al. False-positive TSH receptor antibody—A pitfall of third-generation TSH receptor antibody measurements in neonates. Endocr J. 2018;65(5):587-92. https://pubmed.ncbi.nlm.nih.gov/29526990. https://doi.org/10.1507/endocrj.EJ17-0426.
Briet C, Illouz F, Rodien P. Thyroid hormone receptors. In: Huhtaniemi I, Martini L, eds. Encyclopedia of Endocrine Diseases, 2nd ed. Elsevier; 2019.
Taylor PN, Vaidya B. Side effects of anti-thyroid drugs and their impact on the choice of treatment for thyrotoxicosis in pregnancy. Eur Thyroid J. 2012;1(3):176-85. https://pubmed.ncbi.nlm.nih.gov/24783017. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3821480. https://doi.org/10.1159/000342920.
Lee YS, Loke KY, Ng SCY, Joseph R. Maternal thyrotoxicosis causing central hypothyroidism in infants. 2002;38(2):206-8. https://pubmed.ncbi.nlm.nih.gov/12031010. https://doi.org/10.1046/j.1440-1754.2002.00741.x.
Published
How to Cite
Issue
Section
License
Copyright (c) 2022 Adlina Awanis Mamat @ Abdullah

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
The full license is at this link: http://creativecommons.org/licenses/by-nc/3.0/legalcode).
To obtain permission to translate/reproduce or download articles or use images FOR COMMERCIAL REUSE/BUSINESS PURPOSES from the Journal of the ASEAN Federation of Endocrine Societies, kindly fill in the Permission Request for Use of Copyrighted Material and return as PDF file to jafes@asia.com or jafes.editor@gmail.com.
A written agreement shall be emailed to the requester should permission be granted.










