Patient Characteristics, Disease Burden, Treatment Patterns and Outcomes in Patients with Acromegaly
Real-World Evidence from the Malaysian Acromegaly Registry
DOI:
https://doi.org/10.15605/jafes.038.01.06Keywords:
acromegaly, malaysian registry, healthcare resource utilization, treatment outcomesAbstract
Objective. This study aims to report the demographic features of patients with acromegaly the disease burden, and the corresponding treatment patterns and outcomes in Malaysia.
Methodology. This is a retrospective study that included patients from the Malaysian Acromegaly registry who were diagnosed with acromegaly from 1970 onwards. Data collected included patient demographics, clinical manifestations of acromegaly, biochemical results and imaging findings. Information regarding treatment modalities and their outcomes was also obtained.
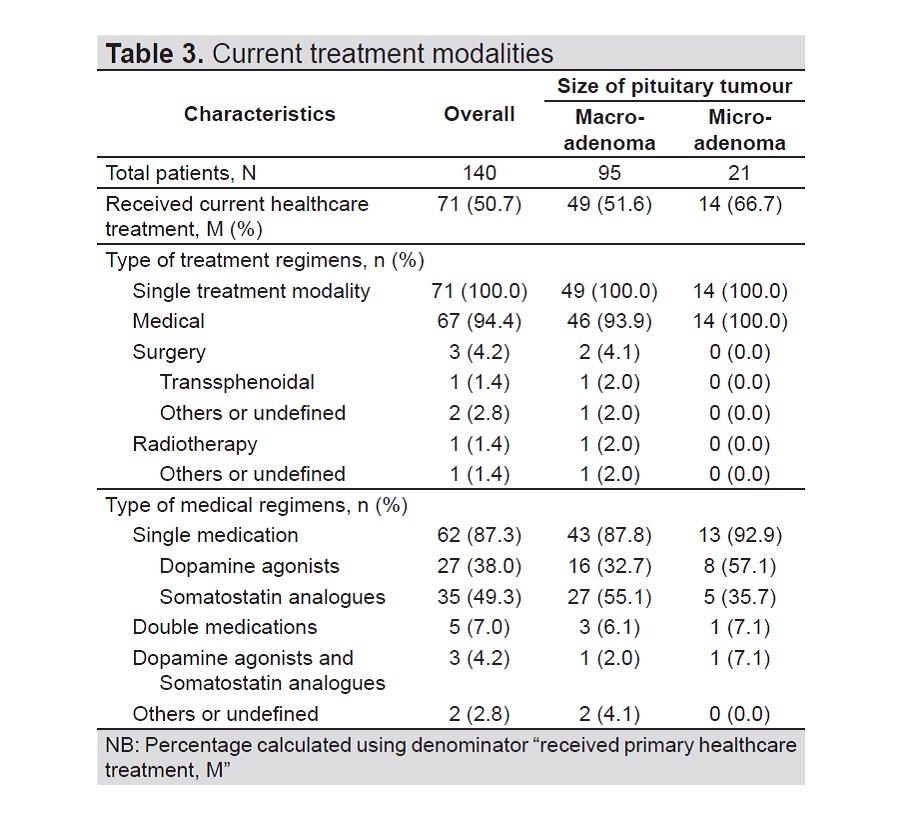
Results. Registry data was collected from 2013 to 2016 and included 140 patients with acromegaly from 12 participating hospitals. Median disease duration was 5.5 years (range 1.0 – 41.0 years). Most patients had macroadenoma (67%), while 15% were diagnosed with microadenoma. Hypertension (49.3%), diabetes (37.1%) and hypopituitarism (27.9%) were the most common co-morbidities for patients with acromegaly. Majority of patients had surgical intervention as primary treatment (65.9%) while 20.7% were treated medically, mainly with dopamine agonists (18.5%). Most patients had inadequate disease control after first-line treatment regardless of treatment modality (79.4%).
Conclusion. This registry study provides epidemiological data on patients with acromegaly in Malaysia and serves as an initial step for further population-based studies.
Downloads
References
Melmed S. Acromegaly pathogenesis and treatment. J Clin Invest. 2009;119:3(11):3189-202. https://pubmed.ncbi.nlm.nih.gov/19884662. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2769196. https://doi.org/10.1172/JCI39375.
Burton T, Le Nestour E, Neary M, Ludlam W. Incidence and prevalence of acromegaly in a large US health plan database. Pituitary. 2016;19(3):262-7. https://pubmed.ncbi.nlm.nih.gov/ 26792654. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4858553. https://doi.org/10.1007/s11102-015-0701-2.
Broder M, Chang E, Cherepanov D, Neary M, Ludlam W. Incidence and prevalence of acromegaly in the United States: A claims-based analysis. Endocr Pract. 2016;22(11):1327-35. https://pubmed.ncbi.nlm.nih.gov/ 27540880. https://doi.org/10.4158/EP161397.OR.
Dal J, Feldt-Rasmussen U, Andersen M, et al. Acromegaly incidence, prevalence, complications and long-term prognosis: A nationwide cohort study. Eur J Endocrinol. 2016;175(3):181-90. https://pubmed.ncbi.nlm.nih.gov/ 27280374. https:/doi.org/10.1530/EJE-16-0117.
Gatto F, Trifirò G, Lapi F, et al. Epidemiology of acromegaly in Italy: Analysis from a large longitudinal primary care database. Endocrine. 2018;61(3):533-41. https://pubmed.ncbi.nlm.nih.gov/ 29797214. https://doi.org/10.1007/s12020-018-1630-4.
Kwon O, Song YD, Seong YK, Lee EJ; for the Rare Disease Study Group, Science and Research Committee, Korean Endocrine Society. Nationwide survey of acromegaly in South Korea. Clin Endocrinol (Oxf). 2013;78(4):577-585. https://pubmed.ncbi.nlm.nih.gov/22909047. https://doi.org/10.1111/cen.12020.
Gadelha MR, Kasuki L, Lim DST, Fleseriu M. Systemic complications of acromegaly and the impact of the current treatment landscape: An update. Endocr Rev. 2019;40(1):268-332. https://pubmed.ncbi.nlm.nih.gov/ 30184064. https://doi.org/10.1210/er.2018-00115.
Colao A, Grasso LFS, Giustina A, et al. Acromegaly. Nat Rev Dis Primers. 2019;5(1):20. https://pubmed.ncbi.nlm.nih.gov/ 30899019. https://doi.org/10.1038/s41572-019-0071-6.
Tirosh A, Shimon I. Complications of acromegaly: Thyroid and colon. Pituitary. 2017;20(1):70-5. https://pubmed.ncbi.nlm.nih.gov/27631334. https://doi.org/10.1007/s11102-016-0744-z.
Pivonello R, Auriemma RS, Grasso LF, et al. Complications of acromegaly: Cardiovascular, respiratory and metabolic comorbidities. Pituitary. 2017;20(1):46-62. https://pubmed.ncbi.nlm.nih.gov/ 28224405. https://doi.org/10.1007/s11102-017-0797-7.
Dineen R, Stewart PM, Sherlock M. Acromegaly. QJM 2017;110(7):411–20. https://pubmed.ncbi.nlm.nih.gov/26873451. https://doi.org/10.1093/qjmed/hcw004.
Nabarro JD. Acromegaly. Clin Endocrinol (Oxf) 1987;26(4):481–512. https://pubmed.ncbi.nlm.nih.gov/3308190. https://doi.org/10.1111/j.1365-2265.1987.tb00805.x.
Ezzat S, Forster MJ, Berchtold P, Redelmeier DA, Boerlin V, Harris AG. Acromegaly. Clinical and biochemical features in 500 patients. Medicine (Baltimore). 1994;73(5):233–40. https://pubmed.ncbi.nlm.nih.gov/ 7934807.
Chanson P, Salenave S. Acromegaly. Orphanet J Rare Dis. 2008;3:17. https://pubmed.ncbi.nlm.nih.gov/18578866. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2459162. https://doi.org/10.1186/1750-1172-3-17.
Esposito D, Ragnarsson O, Johannsson G, Olsson DS. Prolonged diagnostic delay in acromegaly is associated with increased morbidity and mortality. Eur J Endocrinol. 2020;182(6):523-31. https://pubmed.ncbi.nlm.nih.gov/ 32213651. https://doi.org/10.1530/EJE-20-0019.
Kreitschmann-Andermahr I, Buchfelder M, Kleist B, et al. Predictors of quality of life in 165 patients with acromegaly: Results from a single-center study. Endocr Pract. 2017;23(1):79-88. https://pubmed.ncbi.nlm.nih.gov/27749131. https://doi.org/10.4158/EP161373.OR.
Hussein Z, Bidin M, Alias A, et al. Malaysian consensus statement for the diagnosis and management of acromegaly. J ASEAN Fed Endocr Soc. 2019;34(1):8-14. https://pubmed.ncbi.nlm.nih.gov/33442131. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7784186. https://doi.org/10.15605/jafes.034.01.03.
Katznelson L, Laws ER Jr, Melmed S, et al. Acromegaly: An endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2014;99(11):3933-51. https://pubmed.ncbi.nlm.nih.gov/25356808. https://doi.org/10.1210/jc.2014-2700.
Katznelson L, Atkinson JL, Cook DM, et al. American Association of Clinical Endocrinologists medical guidelines for clinical practice for the diagnosis and treatment of acromegaly--2011 update. Endocr Pract. 2011;17(Suppl 4):1-44. https://pubmed.ncbi.nlm.nih.gov/21846616. https://doi.org/10.4158/ep.17.s4.1.
Matsubayashi K, Kawakami K. Prevalence, incidences, comorbidities, and treatment patterns among Japanese patients with acromegaly: A descriptive study using a nationwide claims database. Endocr J. 2020;67(10):997-1006. https://pubmed.ncbi.nlm.nih.gov/32522909. https://doi.org/10.1507/endocrj.EJ20-0129.
Yun SJ, Lee, JK, Park SY, Chin SO. Descriptive epidemiology and survival analysis of acromegaly in Korea. J Korean Med Sci. 2021;36(23):e159. https://pubmed.ncbi.nlm.nih.gov/34128596. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8203854. https://doi.org/10.3346/jkms.2021.36.e159.
Guo X, Wang K, Yu S, Gao L, et al. Patient characteristics, diagnostic delays, treatment patterns, treatment outcomes, comorbidities, and treatment costs of acromegaly in China: A nationwide study. Front Endocrinol (Lausanne). 2020;11:610519. https://pubmed.ncbi.nlm.nih.gov/33335513. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7736552. https://doi.org/10.3389/fendo.2020.610519.
Yuen K, Cook D, Sahasranam P, et al. Prevalence of GH and other anterior pituitary hormone deficiencies in adults with nonsecreting pituitary microadenomas and normal serum IGF-1 levels. Clin Endocrinol (Oxf). 2008;69(2):292-8. https://pubmed.ncbi.nlm.nih.gov/18221393. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2953553. https://doi.org/10.1111/j.1365-2265.2008.03201.x.
Manappallil RG, Veethil PP, Babu H, Khan SR. Pituitary microadenoma with hypopituitarism presenting as hyponatremia. BMJ Case Reports. 2021;14(8):e244426. https://pubmed.ncbi.nlm.nih.gov/34380688. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8359447 (available on 2023-08-11). https://doi.org/10.1136/bcr-2021-244426.
Dal J, Leisner M, Hermansen K, et al. Cancer incidences in patients with acromegaly: A cohort study and meta-analysis of the literature. J Clin Endocrinol Metab. 2018;103(6):2182-8. https://pubmed.ncbi.nlm.nih.gov/29590449. https://doi.org/10.1210/jc.2017-02457.
Tseng F, Huang T, Lin J, Chen S, et al. A registry of acromegaly patients and one year following up in Taiwan. J Formos Med Assoc. 2019;118(10):1430-7. https://pubmed.ncbi.nlm.nih.gov/30612883. https://doi.org/10.1016/j.jfma.2018.12.017.
van der Lely AJ, Hutson RK, Trainer PJ, et al. Long-term treatment of acromegaly with pegvisomant, a growth hormone receptor antagonist. Lancet. 2001;358(9295):1754-9. https://pubmed.ncbi.nlm.nih.gov/11734231. https://doi.org/10.1016/s0140-6736(01)06844-1.
Hannon MJ, Barkan AL, Drake WM. The role of radiotherapy in acromegaly. Neuroendocrinology. 2016;103(1):42-9. https://pubmed.ncbi.nlm.nih.gov/26088716. https://doi.org/10.1159/000435776.
Melmed S, Bronstein MD, Chanson P, et al. A consensus statement on acromegaly therapeutic outcomes. Nat Rev Endocrinol. 2018;14(9):552-61. https://pubmed.ncbi.nlm.nih.gov/30050156. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7136157. https://doi.org/10.1038/s41574-018-0058-5.
Frara S, Maffezzoni F, Mazziotti G, Giustina A. The modern criteria for medical management of acromegaly. Prog Mol Biol Transl Sci. 2016;138:63–83. https://pubmed.ncbi.nlm.nih.gov/26940387. https://doi.org/10.1016/bs.pmbts.2015.10.015.
Bolanowski M, Adnan Z, Doknic M, et al. Acromegaly: Clinical care in Central and Eastern Europe, Israel and Kazakhstan. Front Endocrinol (Lausanne). 2022;13:816426. https://pubmed.ncbi.nlm.nih.gov/35273565. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8902495. https://doi.org/10.3389/fendo.2022.816426.
Christofides E. Clinical importance of achieving biochemical control with medical therapy in adult patients with acromegaly. Patient Prefer Adherence. 2016;10:1217-25. https://pubmed.ncbi.nlm.nih.gov/27471378. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4948729. https://doi.org/10.2147/PPA.S102302.
Published
How to Cite
Issue
Section
License
Copyright (c) 2023 Mohamed Badrulnizam Long Bidin, Florence Hui Sieng Tan, Nor Azizah Aziz, Norhaliza Mohd Ali, Nor Azmi Kamaruddin, Shireene Vethakkan, Abdul Mueed Khan, Balraj Sethi, Zanariah Hussein

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
The full license is at this link: http://creativecommons.org/licenses/by-nc/3.0/legalcode).
To obtain permission to translate/reproduce or download articles or use images FOR COMMERCIAL REUSE/BUSINESS PURPOSES from the Journal of the ASEAN Federation of Endocrine Societies, kindly fill in the Permission Request for Use of Copyrighted Material and return as PDF file to jafes@asia.com or jafes.editor@gmail.com.
A written agreement shall be emailed to the requester should permission be granted.










